
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 7 Lecture Structure and Synthesis of Alkenes Rizalia Klausmeyer Baylor University Waco,TX 2013 Pearson Education,Inc. ALWAYS LEARNING PEARSON
Chapter 7 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Structure and Synthesis of Alkenes © 2013 Pearson Education, Inc. Rizalia Klausmeyer Baylor University Waco, TX 1
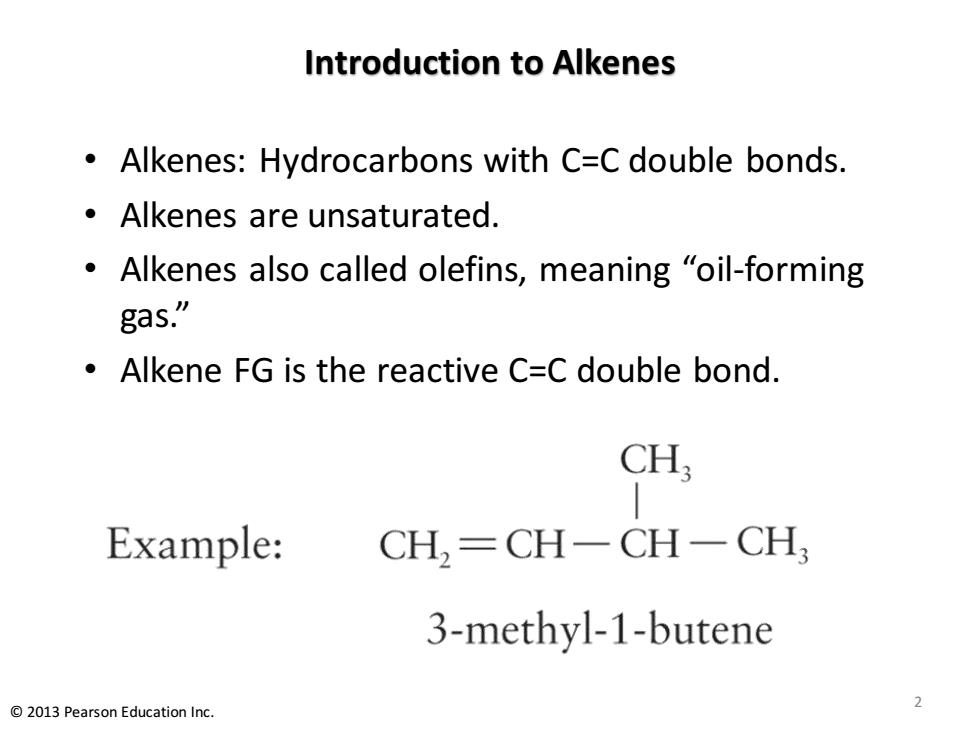
Introduction to Alkenes Alkenes:Hydrocarbons with C=C double bonds. Alkenes are unsaturated. Alkenes also called olefins,meaning "oil-forming gas.” Alkene FG is the reactive C=C double bond. CH; Example: CH,=CH-CH一CH 3-methyl-1-butene 2013 Pearson Education Inc
Introduction to Alkenes • Alkenes: Hydrocarbons with C=C double bonds. • Alkenes are unsaturated. • Alkenes also called olefins, meaning “oil-forming gas.” • Alkene FG is the reactive C=C double bond. © 2013 Pearson Education Inc. 2
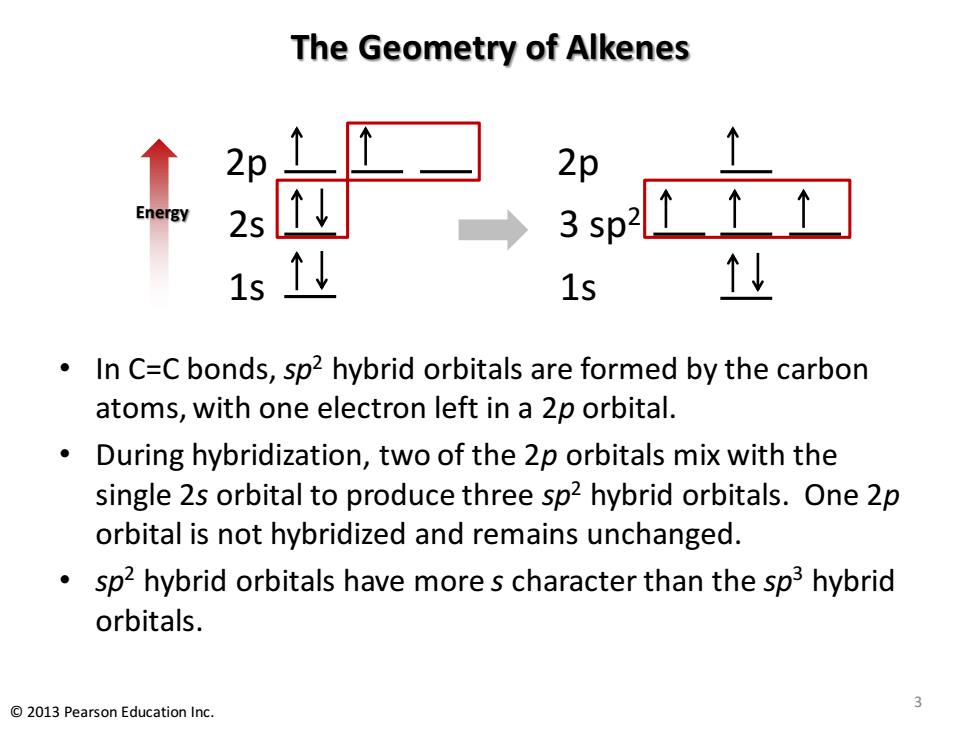
The Geometry of Alkenes 2p 2p Energy 2s 3sp2↑↑↑ 15 1s 型 In C=C bonds,sp2 hybrid orbitals are formed by the carbon atoms,with one electron left in a 2p orbital. During hybridization,two of the 2p orbitals mix with the single 2s orbital to produce three sp2 hybrid orbitals.One 2p orbital is not hybridized and remains unchanged. sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. 3 2013 Pearson Education Inc
The Geometry of Alkenes • In C=C bonds, sp2 hybrid orbitals are formed by the carbon atoms, with one electron left in a 2p orbital. • During hybridization, two of the 2p orbitals mix with the single 2s orbital to produce three sp2 hybrid orbitals. One 2p orbital is not hybridized and remains unchanged. • sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. 2p 2s 1s Energy 2p 3 sp2 1s 3 © 2013 Pearson Education Inc
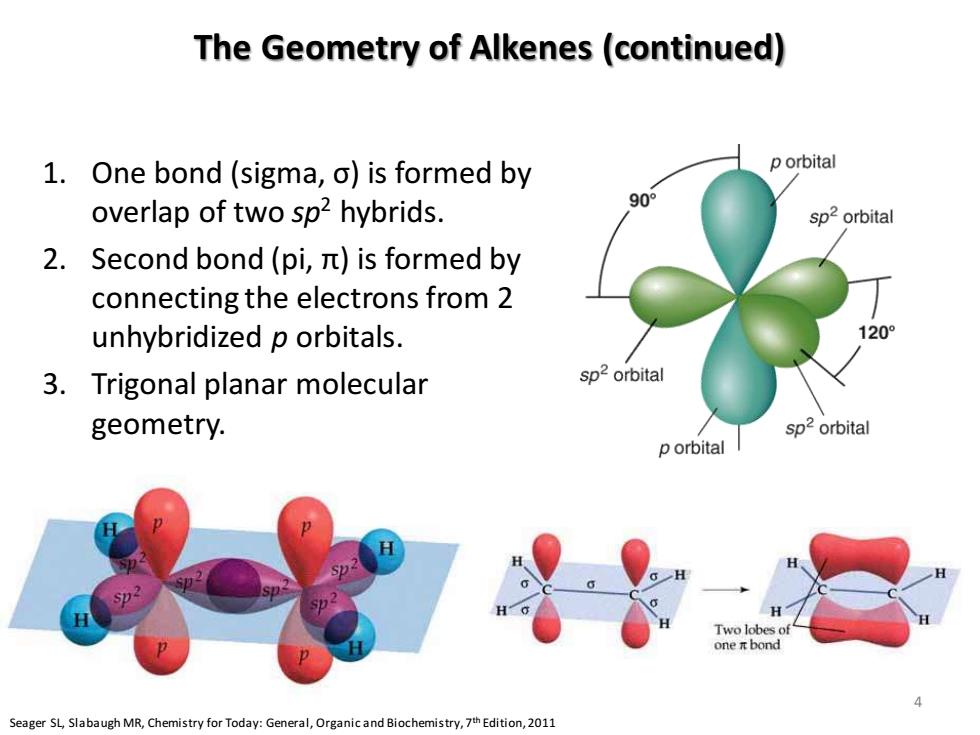
The Geometry of Alkenes(continued) 1.One bond (sigma,o)is formed by p orbital overlap of two sp2 hybrids. 90° sp2orbital 2.Second bond(pi,it)is formed by connecting the electrons from 2 unhybridized p orbitals. 1209 3.Trigonal planar molecular sp2 orbital geometry. sp2 orbital p orbital l H Two lobes of one i bond Seager SL,Slabaugh MR,Chemistry for Today:General,Organic and Biochemistry,7th Edition,2011
The Geometry of Alkenes (continued) 1. One bond (sigma, σ) is formed by overlap of two sp2 hybrids. 2. Second bond (pi, π) is formed by connecting the electrons from 2 unhybridized p orbitals. 3. Trigonal planar molecular geometry. 2p 2s 1s Energy 2p 3 sp2 1s Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 4
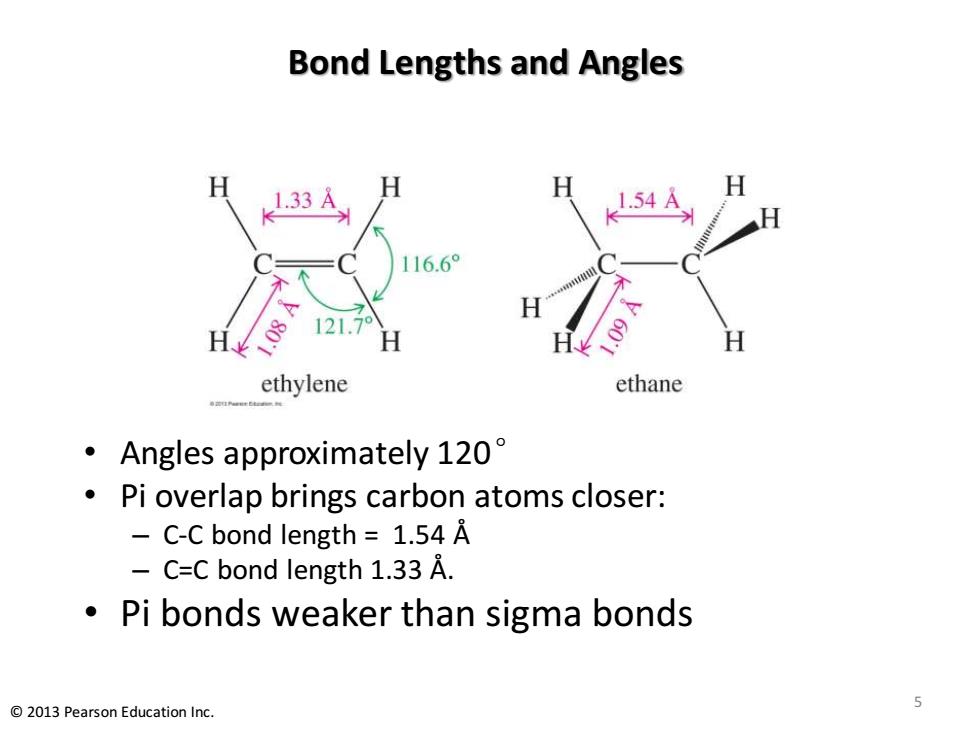
Bond Lengths and Angles H 1.33A 1.54A 116.6° 121.79 ethylene ethane Angles approximately 120 Pi overlap brings carbon atoms closer: C-C bond length 1.54 A C=C bond length 1.33 A. Pi bonds weaker than sigma bonds 5 2013 Pearson Education Inc
Bond Lengths and Angles • Angles approximately 120° • Pi overlap brings carbon atoms closer: – C-C bond length = 1.54 Å – C=C bond length 1.33 Å. • Pi bonds weaker than sigma bonds 5 © 2013 Pearson Education Inc