
Organic Chemistry, ORGANIC 8th Edition CHEMISTRY L.G.Wade,Jr. Chapter 15 Lecture Conjugated Systems, Orbital Symmetry,and Ultraviolet Spectroscopy G.WADE,J R 2013 Pearson Education,Inc. ALWAYS LEARNING PEARSON
© 2013 Pearson Education, Inc. Chapter 15 1 Chapter 15 Lecture Organic Chemistry, 8 th Edition L. G. Wade, Jr. Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy © 2013 Pearson Education, Inc
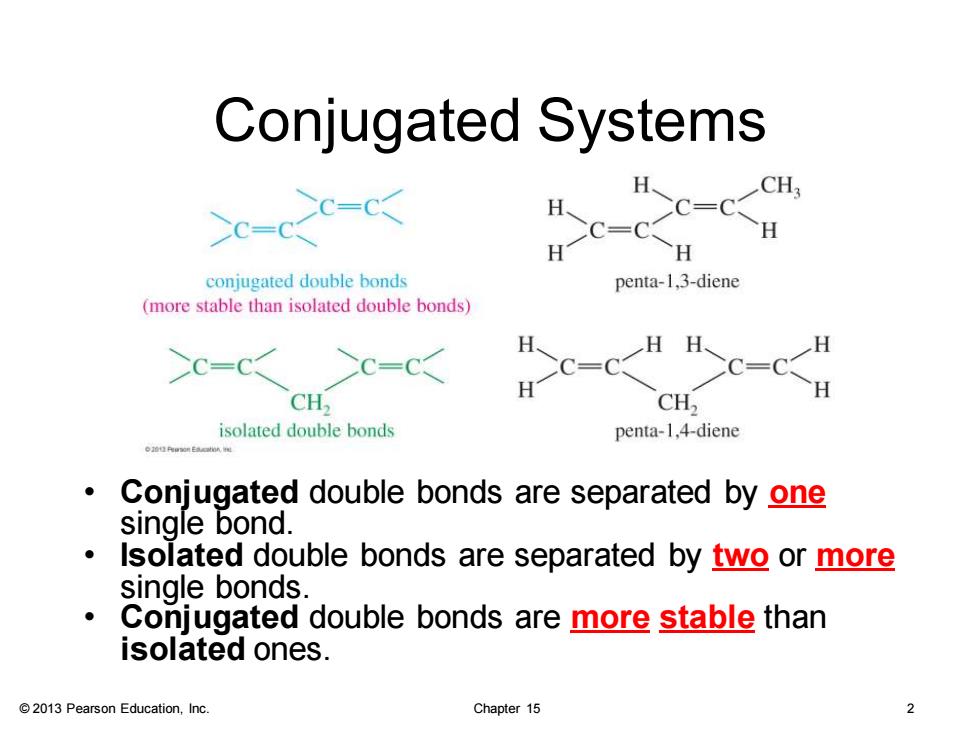
Conjugated Systems c-c-☒ conjugated double bonds penta-1,3-diene (more stable than isolated double bonds) H、 H、 H >c=C< >c=c< CH, CH2 isolated double bonds penta-1,4-diene Conjugated double bonds are separated by one single bond. Isolated double bonds are separated by two or more single bonds Conjugated double bonds are more stable than isolated ones. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 2 Conjugated Systems • Conjugated double bonds are separated by one single bond. • Isolated double bonds are separated by two or more single bonds. • Conjugated double bonds are more stable than isolated ones
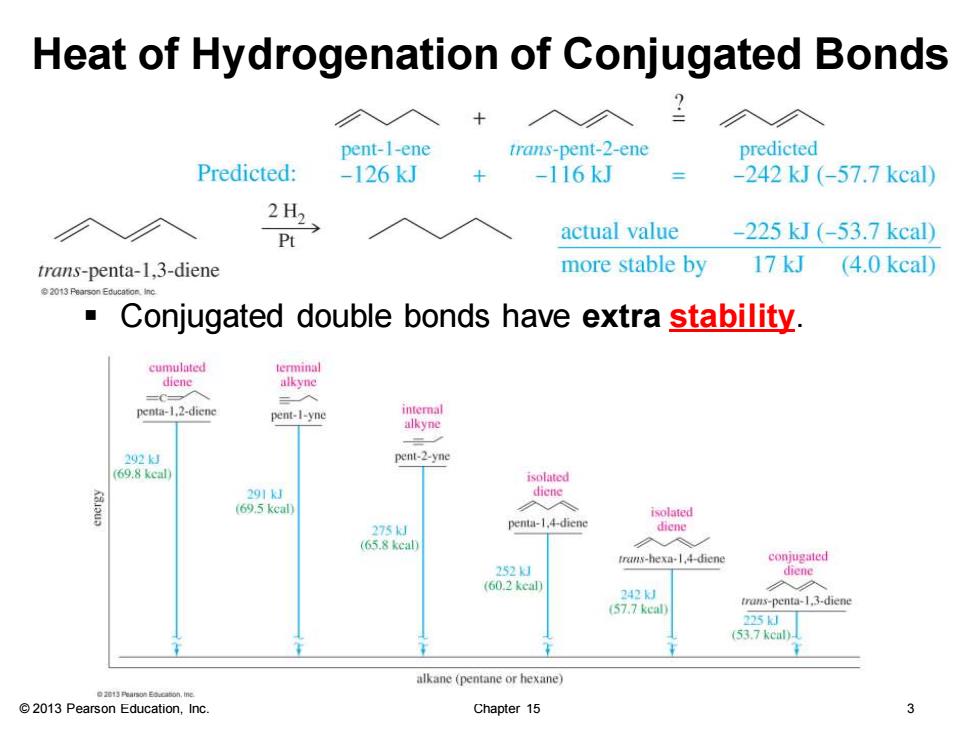
Heat of Hydrogenation of Conjugated Bonds ◇入 pent-1-ene trans-pent-2-ene predicted Predicted: -126kJ -116kJ -242kJ(-57.7kcal) 2H2 Pt actual value -225kJ(-53.7kcal) trans-penta-1,3-diene more stable by 17 kJ (4.0 kcal) Conjugated double bonds have extra stability. cumulated terminal diene alkyne penta-1.2-diene pent-1-yne alkyne 202kJ pent-2-yne (69.8 keal isolated 到 291kJ diene (69.5 keal) isolated 275k penta-1.4-dien diene (65.8 keal) 入 trans-hexa-1,4-dicne conjugated 252」 diene (60.2 keal 242 (57.7 keal trans-penta-1.3-diene 225J (53.7 kcal)- alkane (pentane or hexane) 2013 Pearson Education,Inc. Chapter 15 3
© 2013 Pearson Education, Inc. Chapter 15 3 Heat of Hydrogenation of Conjugated Bonds ▪ Conjugated double bonds have extra stability
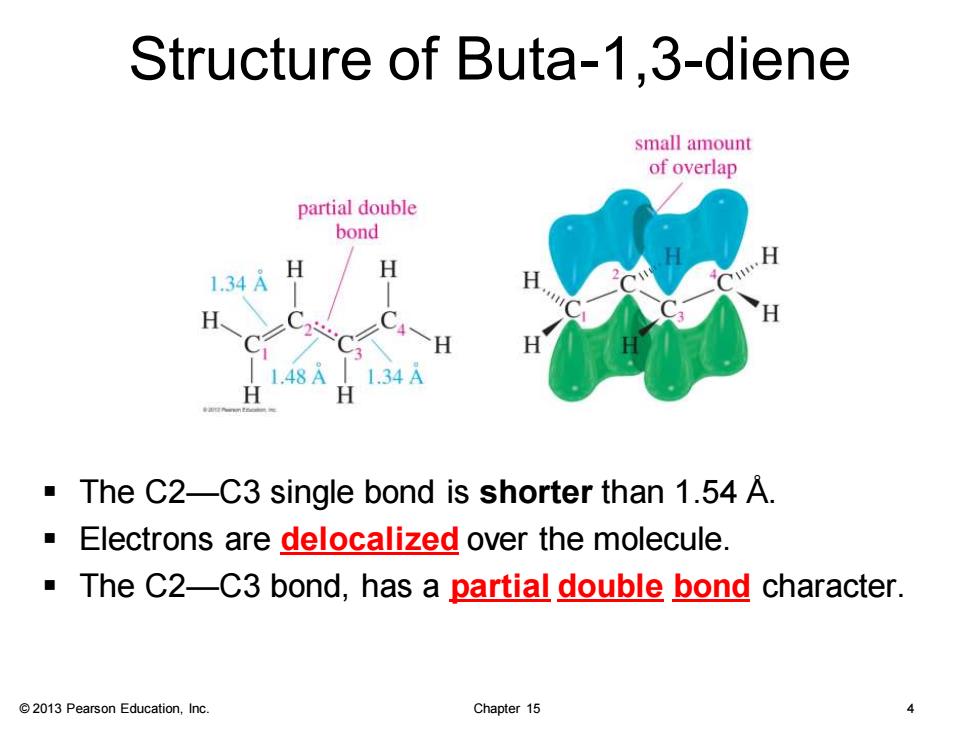
Structure of Buta-1.3-diene small amount of overlap partial double bond H 1.34A 1.48A L.34A The C2-C3 single bond is shorter than 1.54 A. Electrons are delocalized over the molecule. The C2-C3 bond,has a partial double bond character. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 4 Structure of Buta-1,3-diene ▪ The C2—C3 single bond is shorter than 1.54 Å. ▪ Electrons are delocalized over the molecule. ▪ The C2—C3 bond, has a partial double bond character
Molecular Orbitals(MOs) -Each p orbital has two lobes with the wave function indicated by plus (+)and minus(-) sign (not electrical charges). -When lobes overlap constructively (and + or-and-),a pi bonding (MO is formed. -When lobes overlap destructively (and-), a pi antibonding(π*)MO is formed. 2013 Pearson Education,Inc. Chapter 15 5
© 2013 Pearson Education, Inc. Chapter 15 5 Molecular Orbitals (MOs) ▪ Each p orbital has two lobes with the wave function indicated by plus (+) and minus (–) sign (not electrical charges). ▪ When lobes overlap constructively (+ and +, or – and –), a pi bonding (p) MO is formed. ▪ When lobes overlap destructively (+ and –), a pi antibonding (p*) MO is formed