
E2 Stereochemistry Base: Basc一H R 0=1809 anti-coplanar transition state (staggered conformation-lower energy) Base: repulsion Base—H X R R 0=0° syn-coplanar transition state (eclipsed conformation-higher energy) Chapter 6 21
E2 Stereochemistry Chapter 6 21

E1 or E2 Mechanism? 。Tertiary>secondary ·Tertiary>secondary Base strength unimportant 。Strong base required (usually weak) Good ionizing solvent ·Solvent polarity not important. Rate k[alkyl halide] Rate k[alkylhalide][base] ·Zaitsev product ·Zaitsev product ·No required geometry Coplanar leaving groups (usually anti) ·Rearranged products 。No rearrangements Chapter 6 22
E1 or E2 Mechanism? • Tertiary > secondary • Base strength unimportant (usually weak) • Good ionizing solvent • Rate = k[alkyl halide] • Zaitsev product • No required geometry • Rearranged products • Tertiary > secondary • Strong base required • Solvent polarity not important. • Rate = k[alkylhalide][base] • Zaitsev product • Coplanar leaving groups (usually anti) • No rearrangements Chapter 6 22

Substitution or Elimination? Strength of the nucleophile determines order:Strong nucleophiles or bases promote bimolecular reactions. Primary halides usually undergo Tertiary halides are a mixture of or.They cannot undergo SN2. Elimination is favored by: High temperature Bulky bases. Chapter 6 23
Substitution or Elimination? • Strength of the nucleophile determines order: Strong nucleophiles or bases promote bimolecular reactions. • Primary halides usually undergo _____. • Tertiary halides are a mixture of ____, ___, or ____. They cannot undergo SN2. • Elimination is favored by: – High temperature – Bulky bases. . Chapter 6 23
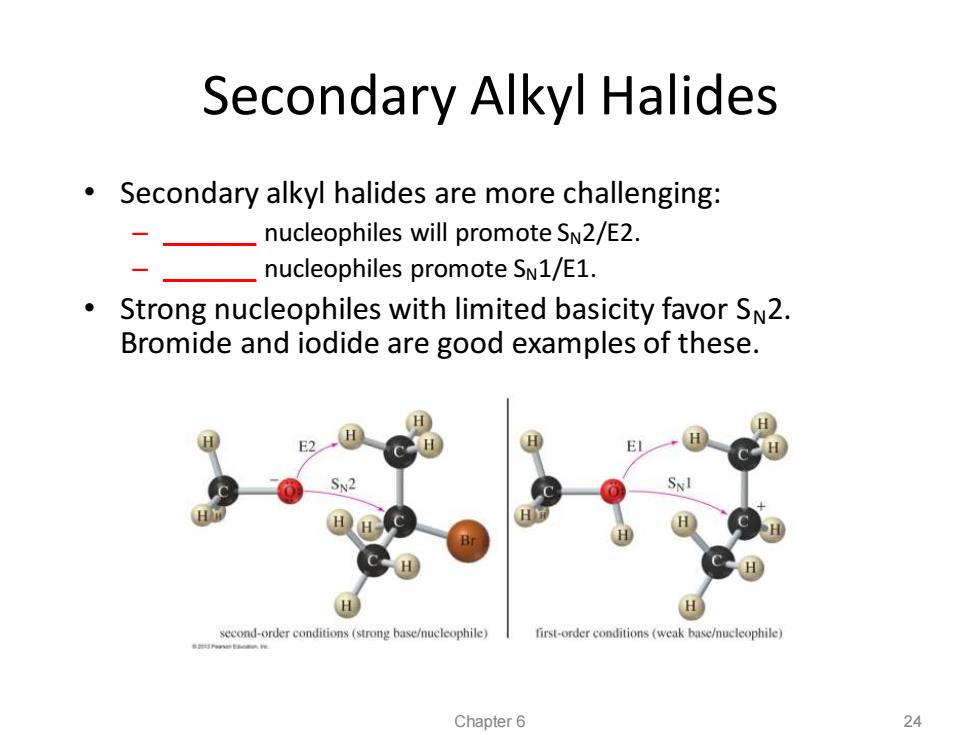
Secondary Alkyl Halides Secondary alkyl halides are more challenging: -nucteophiles will promote SN2/E2. nucleophiles promote SN1/E1. Strong nucleophiles with limited basicity favor SN2. Bromide and iodide are good examples of these. H SN2 H H H second-order conditions(strong base/nucleophile) first-order conditions (weak base/nucleophile) Chapter 6 24
Secondary Alkyl Halides • Secondary alkyl halides are more challenging: – _______ nucleophiles will promote SN2/E2. – _______ nucleophiles promote SN1/E1. • Strong nucleophiles with limited basicity favor SN2. Bromide and iodide are good examples of these. Chapter 6 24

Hofmann Product Bulky bases (like t-butoxide)abstract the H+ 。 the major product is the substituted alkene ·It is called the product. Non-bulky bases(like ethoxide)abstract the H+ the major product is the substituted alkene It is called the product. Zaitsey product Hofmann product CH3 H.C -OCH,CH3 CH CH3一CH2 CH3-C-C-CH2 CHCH,OH H Br H CH H.C 71% 29% 2013 Pearson Education.inc Chapter 7 25
Hofmann Product • Bulky bases (like t-butoxide) abstract the _______ __________ H + • the major product is the _____ substituted alkene • It is called the _________ product. Chapter 7 25 • Non-bulky bases (like ethoxide) abstract the ______ __________ H+ • the major product is the ______ substituted alkene • It is called the ________ product