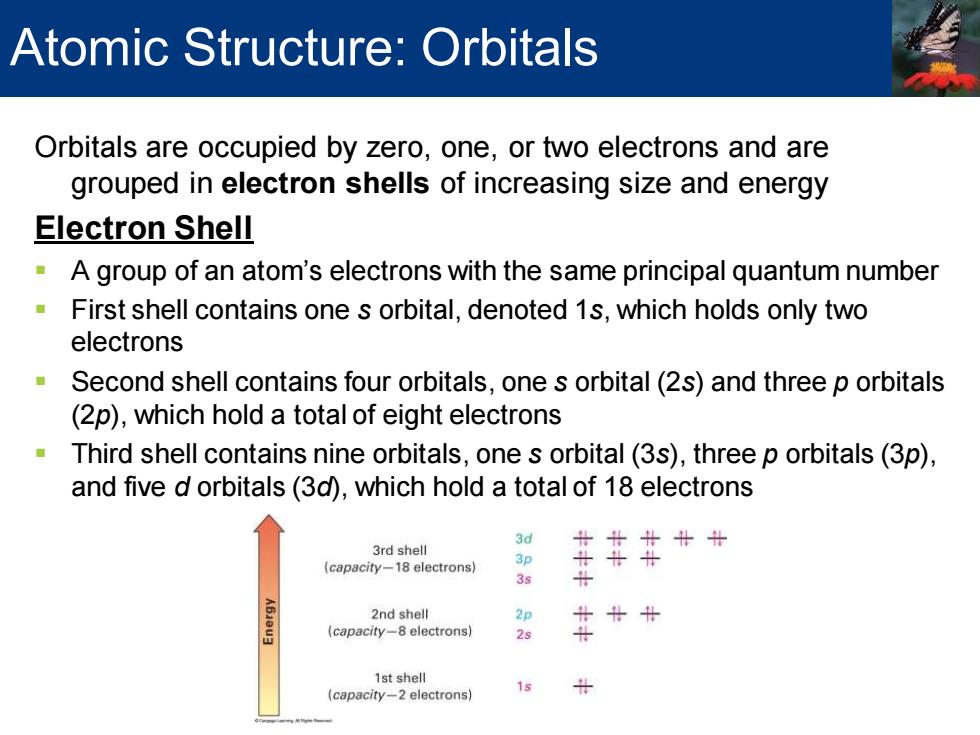
Atomic Structure:Orbitals Orbitals are occupied by zero,one,or two electrons and are grouped in electron shells of increasing size and energy Electron Shell A group of an atom's electrons with the same principal quantum number -First shell contains one s orbital,denoted 1s,which holds only two electrons Second shell contains four orbitals,one s orbital(2s)and three p orbitals (2p),which hold a total of eight electrons Third shell contains nine orbitals,one s orbital(3s),three p orbitals(3p), and five d orbitals(3d),which hold a total of 18 electrons 3d 3rd shell 北北计计计 (capacity-18 electrons) 3s 2nd shell 2p (capacity-8 electrons) 2s 1st shell 19 (capacity-2 electrons)
Orbitals are occupied by zero, one, or two electrons and are grouped in electron shells of increasing size and energy Electron Shell ▪ A group of an atom’s electrons with the same principal quantum number ▪ First shell contains one s orbital, denoted 1s, which holds only two electrons ▪ Second shell contains four orbitals, one s orbital (2s) and three p orbitals (2p), which hold a total of eight electrons ▪ Third shell contains nine orbitals, one s orbital (3s), three p orbitals (3p), and five d orbitals (3d), which hold a total of 18 electrons Atomic Structure: Orbitals
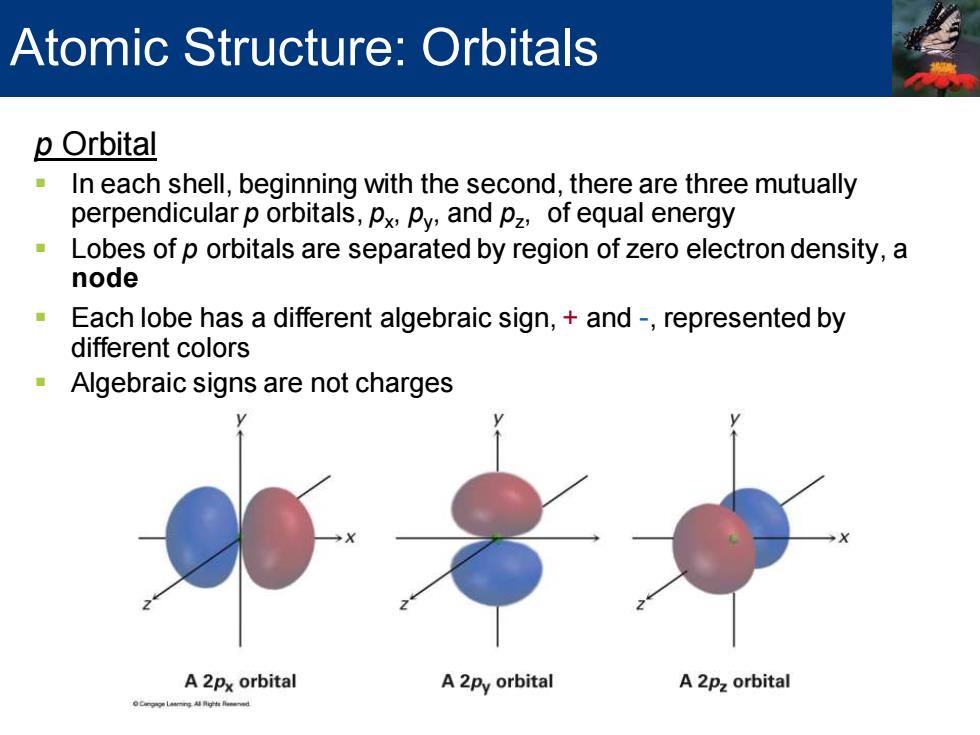
Atomic Structure:Orbitals p Orbital In each shell,beginning with the second,there are three mutually perpendicular p orbitals,px,py,and pz,of equal energy Lobes of p orbitals are separated by region of zero electron density,a node Each lobe has a different algebraic sign,and-,represented by different colors Algebraic signs are not charges A 2px orbital A 2py orbital A 2pz orbital oCgg年4ngh
p Orbital ▪ In each shell, beginning with the second, there are three mutually perpendicular p orbitals, px , py , and pz , of equal energy ▪ Lobes of p orbitals are separated by region of zero electron density, a node ▪ Each lobe has a different algebraic sign, + and -, represented by different colors ▪ Algebraic signs are not charges Atomic Structure: Orbitals
1-3 Atomic Structure:Electron Configuration Ground-state electron configuration Most stable,lowest-energy electron configuration of an atom Three rules: 1.Aufbau principle ·Lowest-.energy orbitals fill first:1s-→2s→2p-→3s→3p→4s-→ 3d 2.Pauli Exclusion Principle Electron spin can have only two orientations,up T and down Only two electrons can occupy an orbital,and they must be of opposite spin to have unique wave equations
Ground-state electron configuration ▪ Most stable, lowest-energy electron configuration of an atom Three rules: 1. Aufbau principle ▪ Lowest-energy orbitals fill first: 1s → 2s → 2p → 3s → 3p → 4s → 3d 2. Pauli Exclusion Principle ▪ Electron spin can have only two orientations, up and down ▪ Only two electrons can occupy an orbital, and they must be of opposite spin to have unique wave equations 1-3 Atomic Structure: Electron Configuration
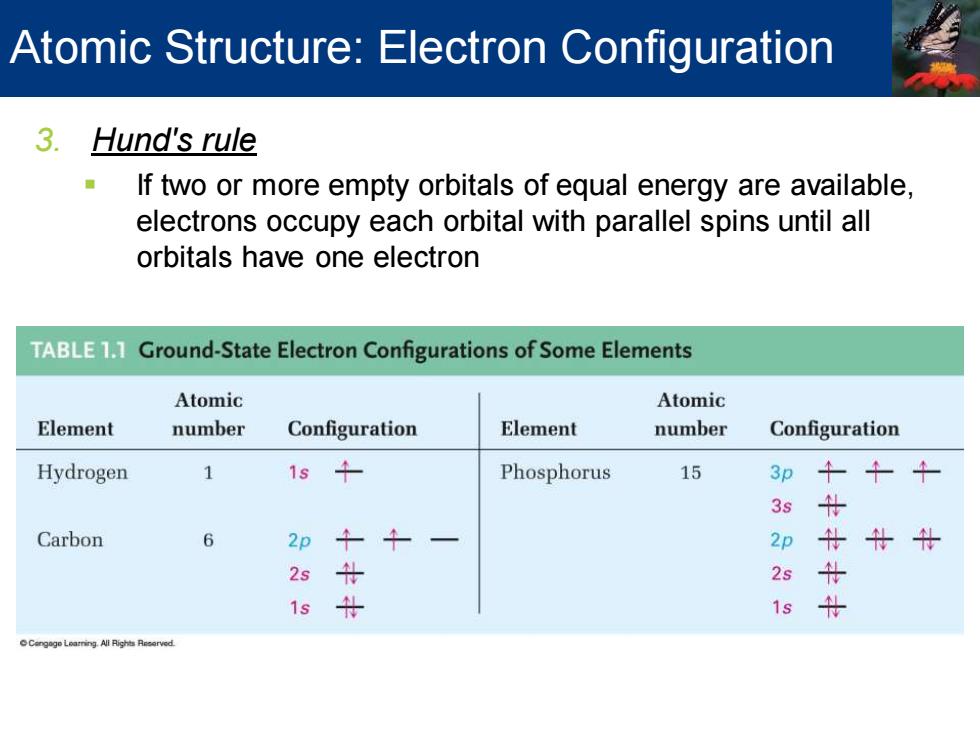
Atomic Structure:Electron Configuration 3. Hund's rule If two or more empty orbitals of equal energy are available, electrons occupy each orbital with parallel spins until all orbitals have one electron TABLE11 Ground-State Electron Configurations of Some Elements Atomic Atomic Element number Configuration Element number Configuration Hydrogen 1 1s 午 Phosphorus 15 3p 3s 什 Carbon 6 2p 2p 什女什 2s 2s 0 1s 1s
3. Hund's rule ▪ If two or more empty orbitals of equal energy are available, electrons occupy each orbital with parallel spins until all orbitals have one electron Atomic Structure: Electron Configuration
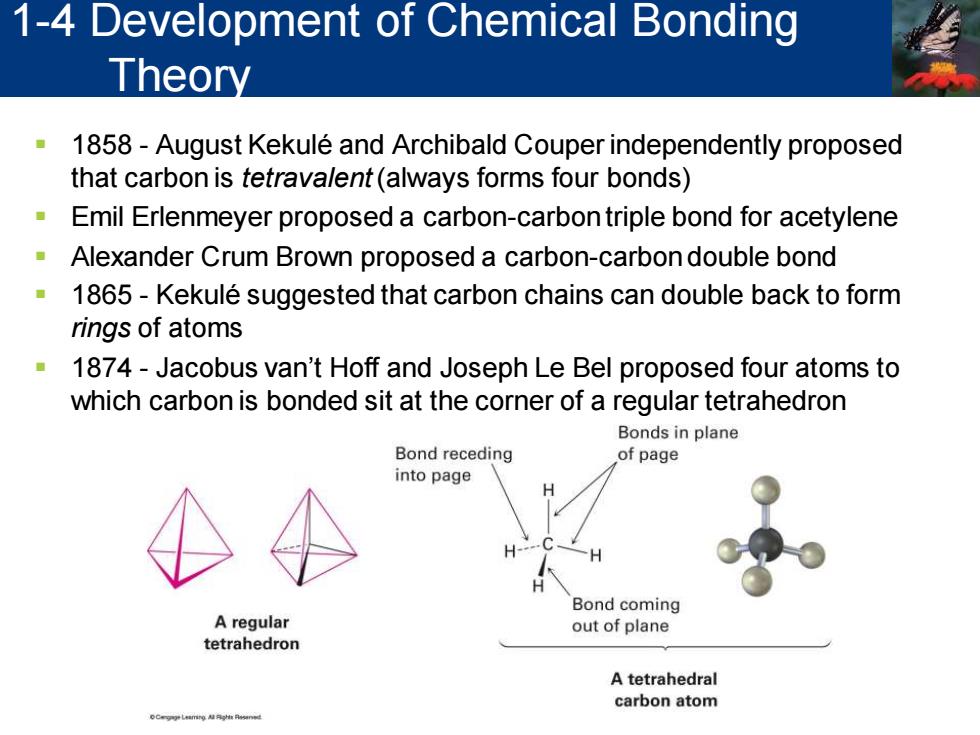
1-4 Development of Chemical Bonding Theory 1858-August Kekule and Archibald Couper independently proposed that carbon is tetravalent(always forms four bonds) Emil Erlenmeyer proposed a carbon-carbon triple bond for acetylene Alexander Crum Brown proposed a carbon-carbon double bond 1865-Kekule suggested that carbon chains can double back to form rings of atoms 1874-Jacobus van't Hoff and Joseph Le Bel proposed four atoms to which carbon is bonded sit at the corner of a regular tetrahedron Bonds in plane Bond receding of page into page Bond coming A regular out of plane tetrahedron A tetrahedral carbon atom
▪ 1858 - August Kekuléand Archibald Couper independently proposed that carbon is tetravalent (always forms four bonds) ▪ Emil Erlenmeyer proposed a carbon-carbon triple bond for acetylene ▪ Alexander Crum Brown proposed a carbon-carbon double bond ▪ 1865 - Kekulé suggested that carbon chains can double back to form rings of atoms ▪ 1874 - Jacobus van’t Hoff and Joseph Le Bel proposed four atoms to which carbon is bonded sit at the corner of a regular tetrahedron 1-4 Development of Chemical Bonding Theory