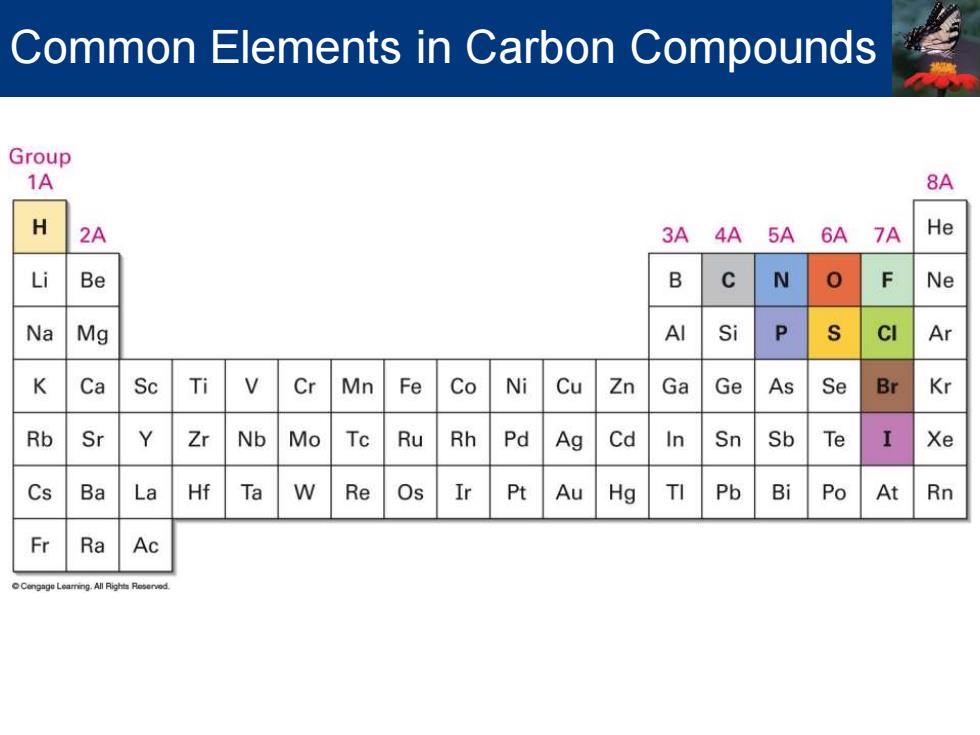
Common Elements in Carbon Compounds Group 1A 8A H 2A 3A 4A 5A 6A 7A He Li Be B N Ne Na Mg AI Si P CI Ar K Ca Sc Ti Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac
Common Elements in Carbon Compounds
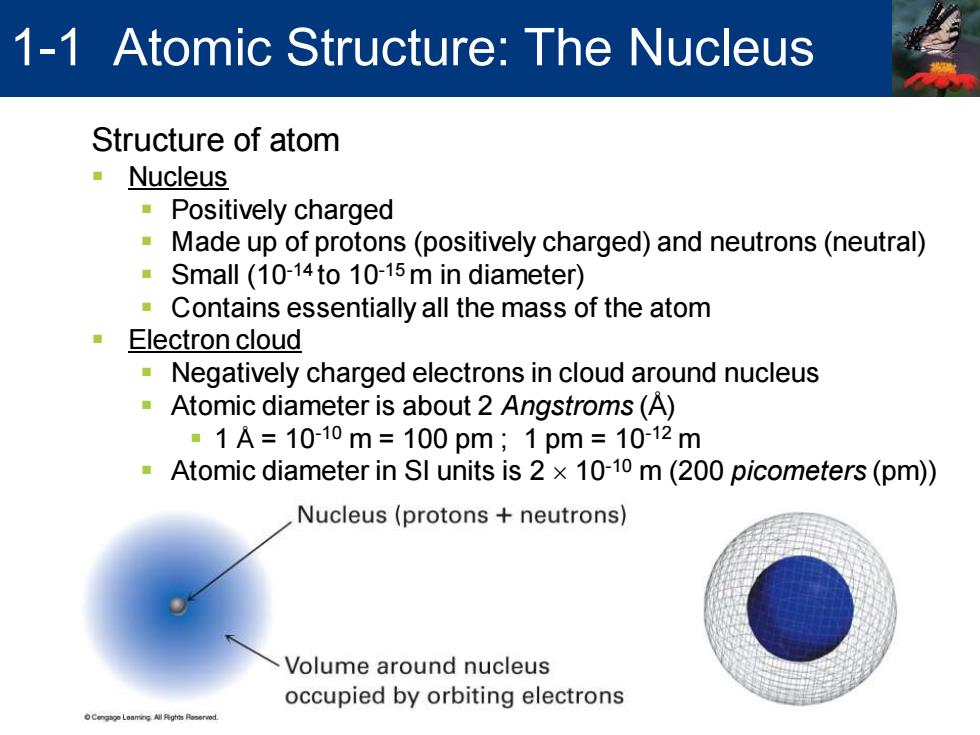
1-1 Atomic Structure:The Nucleus Structure of atom ·Nucleus ·Positively charged Made up of protons(positively charged)and neutrons(neutral) 意 Small (10-14to 10-15m in diameter) Contains essentially all the mass of the atom Electron cloud Negatively charged electrons in cloud around nucleus Atomic diameter is about 2 Angstroms(A) 。1A=1010m=100pm;1pm=1012m Atomic diameter in SI units is 2 x 10-10 m(200 picometers(pm)) Nucleus(protons neutrons) Volume around nucleus occupied by orbiting electrons
Structure of atom ▪ Nucleus ▪ Positively charged ▪ Made up of protons (positively charged) and neutrons (neutral) ▪ Small (10-14 to 10-15 m in diameter) ▪ Contains essentially all the mass of the atom ▪ Electron cloud ▪ Negatively charged electrons in cloud around nucleus ▪ Atomic diameter is about 2 Angstroms (Å) ▪ 1 Å = 10-10 m = 100 pm ; 1 pm = 10-12 m ▪ Atomic diameter in SI units is 2 10-10 m (200 picometers (pm)) 1-1 Atomic Structure: The Nucleus
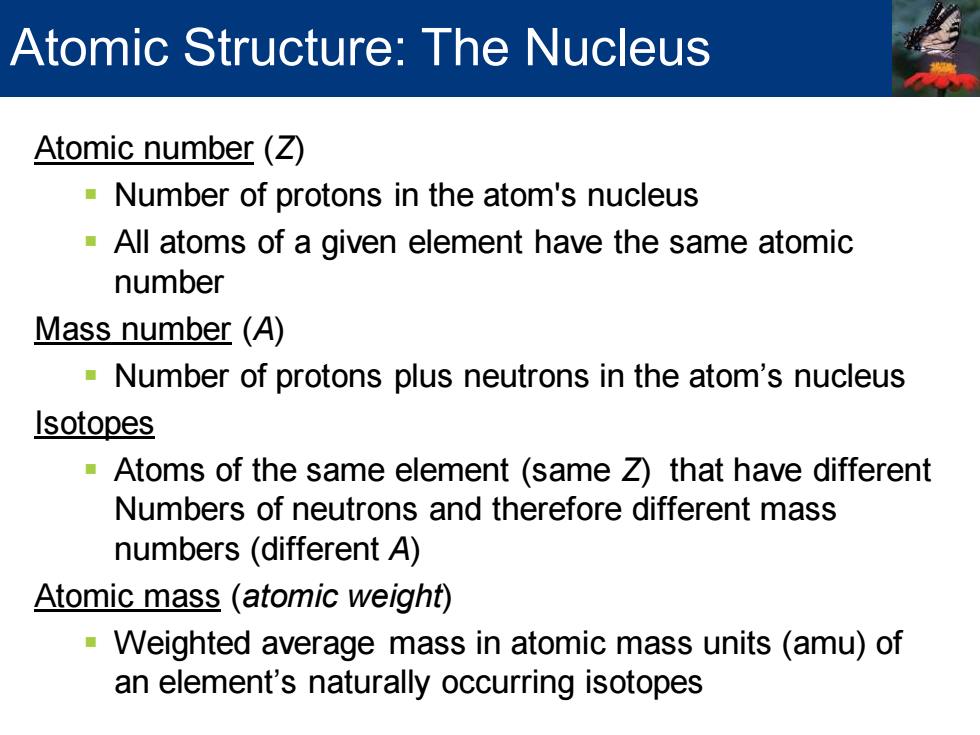
Atomic Structure:The Nucleus Atomic number (Z) Number of protons in the atom's nucleus All atoms of a given element have the same atomic number Mass number (A) Number of protons plus neutrons in the atom's nucleus Isotopes Atoms of the same element (same Z)that have different Numbers of neutrons and therefore different mass numbers(different A) Atomic mass (atomic weight) -Weighted average mass in atomic mass units (amu)of an element's naturally occurring isotopes
Atomic number (Z) ▪ Number of protons in the atom's nucleus ▪ All atoms of a given element have the same atomic number Mass number (A) ▪ Number of protons plus neutrons in the atom’s nucleus Isotopes ▪ Atoms of the same element (same Z) that have different Numbers of neutrons and therefore different mass numbers (different A) Atomic mass (atomic weight) ▪ Weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes Atomic Structure: The Nucleus
1-2 Atomic Structure:Orbitals Quantum Mechanical Model Behavior of a specific electron in an atom described by mathematical expression called a wave equation Wave equation is similar to mathematical expression used to describe motion of waves in fluids -Solution of wave equation is called a wave function -Wave function is an orbital Orbital denoted by Greek letter psi, Plot of 2 describes volume of space around nucleus that the electron is most likely to occupy Electron cloud has no sharp boundary
Quantum Mechanical Model ▪ Behavior of a specific electron in an atom described by mathematical expression called a wave equation ▪ Wave equation is similar to mathematical expression used to describe motion of waves in fluids ▪ Solution of wave equation is called a wave function ▪ Wave function is an orbital ▪ Orbital denoted by Greek letter psi, ▪ Plot of 2 describes volume of space around nucleus that the electron is most likely to occupy ▪ Electron cloud has no sharp boundary 1-2 Atomic Structure: Orbitals
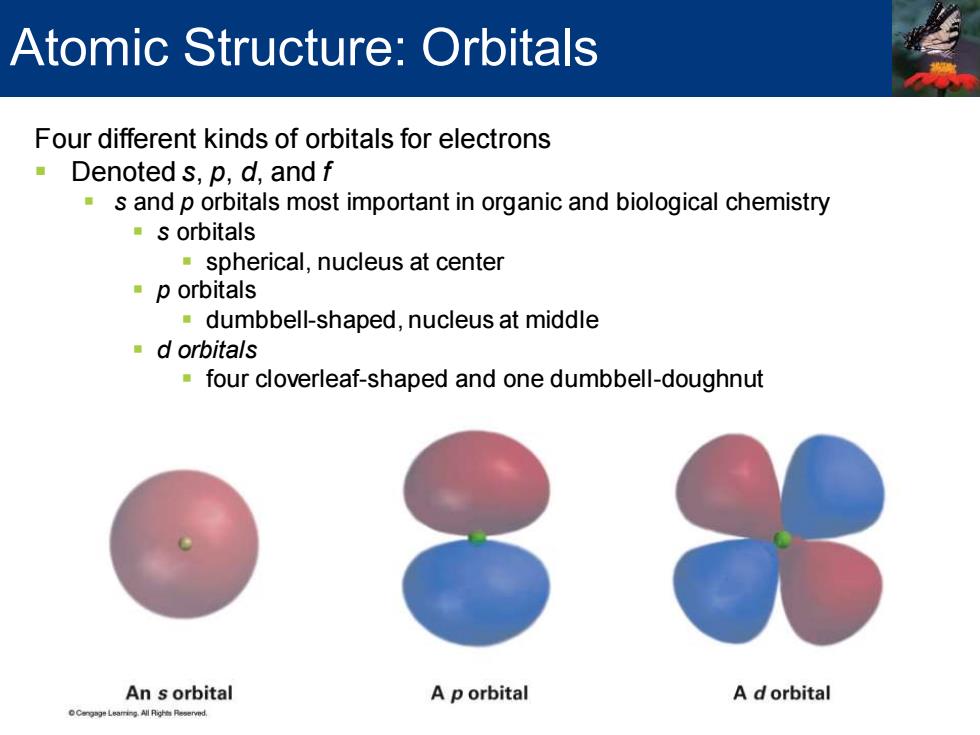
Atomic Structure:Orbitals Four different kinds of orbitals for electrons Denoted s,p,d,and f s and p orbitals most important in organic and biological chemistry s orbitals spherical,nucleus at center p orbitals dumbbell-shaped,nucleus at middle d orbitals four cloverleaf-shaped and one dumbbell-doughnut An s orbital A p orbital A dorbital
Four different kinds of orbitals for electrons ▪ Denoted s, p, d, and f ▪ s and p orbitals most important in organic and biological chemistry ▪ s orbitals ▪ spherical, nucleus at center ▪ p orbitals ▪ dumbbell-shaped, nucleus at middle ▪ d orbitals ▪ four cloverleaf-shaped and one dumbbell-doughnut Atomic Structure: Orbitals