
3)羰基合成 CHCH=CH2 +CO +H2 钴催化剂 CH3CH2CH2CHO +CH3CHCHO 130℃175℃ 25 MPa CH3 烯烃 C0+H2 催化剂 醛 NiC CHCH2CH2CH2OH +CHCHCH2OH △,5MPa CH3 还原 醇
(3) 羰基合成 还原 +CH3CHCHO CH3 CH3CH CH2 + CO + H2 钴催化剂 130℃~175℃ ~25 MPa CH3CH2CH2CHO H2 , Ni 或 Cu △,~5 MPa CH3CH2CH2CH2OH +CH3CHCH2OH CH3 烯烃 CO + H2 催化剂 醛 醇

9.3.2酚的工业制法 (1)从异丙苯制备 ; CH;CH=CH2 H3P04,2500C CH 02,Na2C0 2.41MPa 90-130℃ OH 12%H2S04 6065℃ OH +CH3COCH3 CH3
9.3.2 酚的工业制法 + CH3CH=CH2 H3 PO4 ,250 oC 2.41MPa CH CH3 CH3 90-130 C 0 O2 ,Na2 CO3 C CH3 CH3 OOH 1~2%H2 SO4 60~65 0 C OH + CH3 COCH3 (1)从异丙苯制备
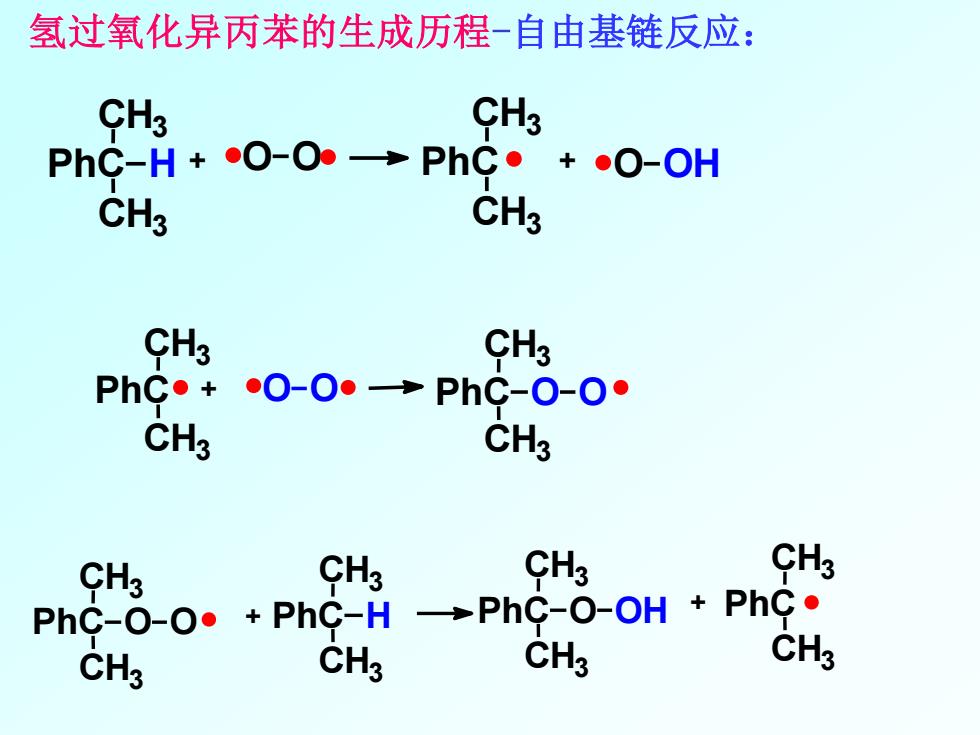
氢过氧化异丙苯的生成历程-自由基链反应: CH3 CH3 PhC-H+O-O→PhC。+O-OH CH3 CH3 CH3 CH3 PhC·+O-0·→PhC-o-o° CH3 CH3 CHg CH3 CHs CH3 PhC-O-O·+PhC-H →PhC-O-Oh+PhC· CH3 CH3 CH3 CH3
氢过氧化异丙苯的生成历程-自由基链反应: PhC CH3 CH3 H + O O PhC + CH3 CH3 O OH PhC CH3 CH3 PhC O O CH3 CH3 + O O + PhC CH3 CH3 PhC H CH3 CH3 O O PhC CH3 CH3 O OH PhC CH3 CH3 +

制备苯酚和丙酮的反应历程-重排: CH3 PhGS-OH CC-Pm-HC-Ph] CHg CH3 CH3+ CH3 Ph CH3. CH3 H.C8月Ph-hc6gpn +OH2 *QH PhOH CH3-C.CH3 L→CHs-C-CHa+t
PhC CH3 CH3 O OH H + C CH3 Ph H3C O OH2 + H2O _ C CH3 H3C O Ph + + C CH3 H3C O Ph H2O C CH3 H3C O Ph + OH2 + C CH3 H3C O Ph OH H PhOH + CH3 C CH3 OH + CH3 C CH3 O + H + 制备苯酚和丙酮的反应历程-重排:

(2)芳卤衍生物的水解 ONa OH No2Na0H,140-155G NO2 H2SO4 NO 450-530kPa,5.5h (3)碱熔法 96%H2S04 SO3H Na2CO3 165℃ SO3Na ONa NaOH 300-320℃ OH
Cl NO2 NaOH, 140-155 450-530kPa, 5.5h 0 C ONa NO2 H2 SO4 OH NO2 96%H2 SO4 165 C 0 SO3H Na2 CO3 SO3Na NaOH 300-320 C 0 ONa CO2 ,H2O OH (2)芳卤衍生物的水解 (3)碱熔法