
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 20 Carboxylic Acids Copyright 2010 Pearson Education,Inc
Chapter 20 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Carboxylic Acids
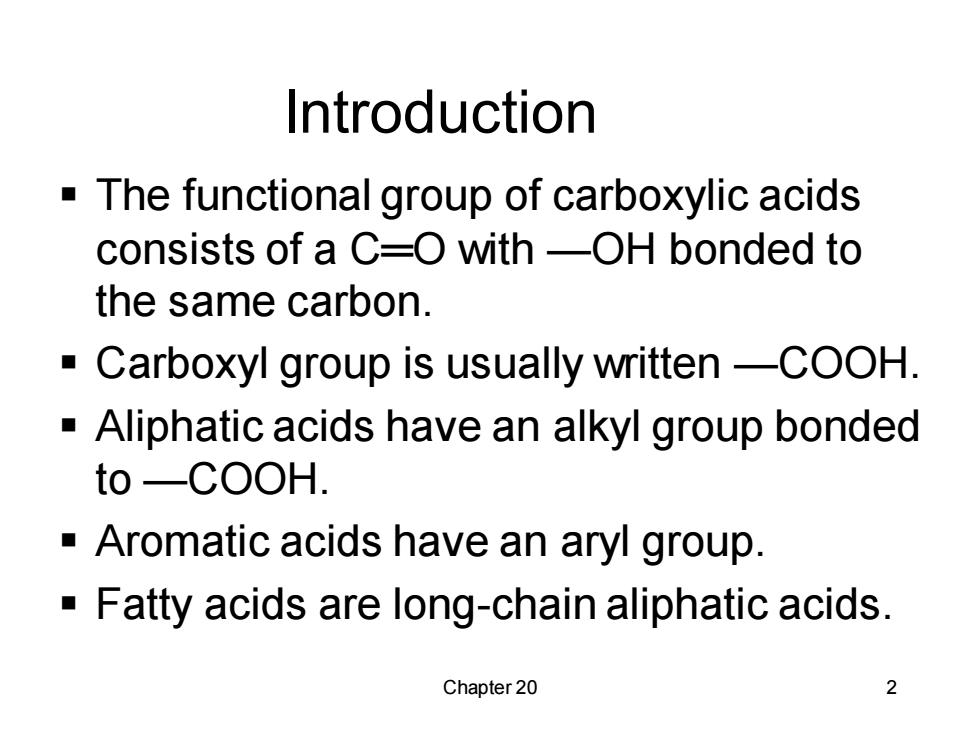
Introduction The functional group of carboxylic acids consists of a C=O with-OH bonded to the same carbon. -Carboxyl group is usually written-COOH. -Aliphatic acids have an alkyl group bonded to -COOH. -Aromatic acids have an aryl group. -Fatty acids are long-chain aliphatic acids. Chapter 20 2
Chapter 20 2 Introduction ▪ The functional group of carboxylic acids consists of a C═O with —OH bonded to the same carbon. ▪ Carboxyl group is usually written —COOH. ▪ Aliphatic acids have an alkyl group bonded to —COOH. ▪ Aromatic acids have an aryl group. ▪ Fatty acids are long-chain aliphatic acids

Common Names es-ccc8om NH, CH CH3-CH-C-OH CH,一CH2一CH2一C-OH CH,一CH一CH2一C-OH B y B a-chloropropionic acid y-aminobutyric acid isovaleric acid (B-methylbutyric acid) Copyright 2010 Pearson Prentice Hall,Inc. Many aliphatic acids have historical names. Positions of substituents on the chain are labeled with Greek letters starting at the carbon attached to the carboxylic carbon Chapter 20 3
Chapter 20 3 Common Names ▪ Many aliphatic acids have historical names. ▪ Positions of substituents on the chain are labeled with Greek letters starting at the carbon attached to the carboxylic carbon
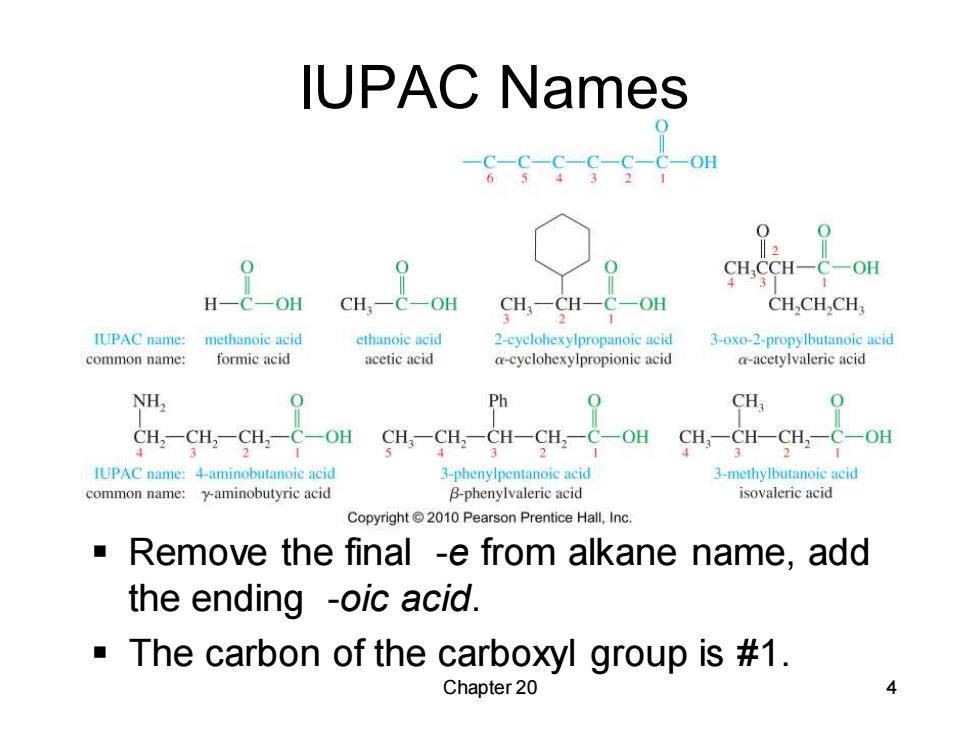
IUPAC Names C-OH ‖2 CHCCH-C-OH H-C-OH CHC一OH CH,-CH-C-OH CH,CH.CH; IUPAC name: methanoic acid ethanoie acid 2-cyelohexylpropanoic acid 3-oxo-2-propylbutanoic acid common name: formic acid acetic acid a-cyclohexylpropionic acid a-acetylvaleric acid NH, 0 Ph 0 CH CH,一CH,一CH,一C-OH CH,一CH,一CH-CH,一C-OH CH,-CH-CH,一C-OH IUPAC name:4-aminobutanoic acid 3-phenylpentanoic acid 3-methylbutanoie acid common name:y-aminobutyric acid B-phenylvaleric acid isovaleric acid Copyright2010 Pearson Prentice Hall,Inc. Remove the final -e from alkane name,add the ending -oic acid. The carbon of the carboxyl group is #1. Chapter 20
Chapter 20 4 IUPAC Names ▪ Remove the final -e from alkane name, add the ending -oic acid. ▪ The carbon of the carboxyl group is #1
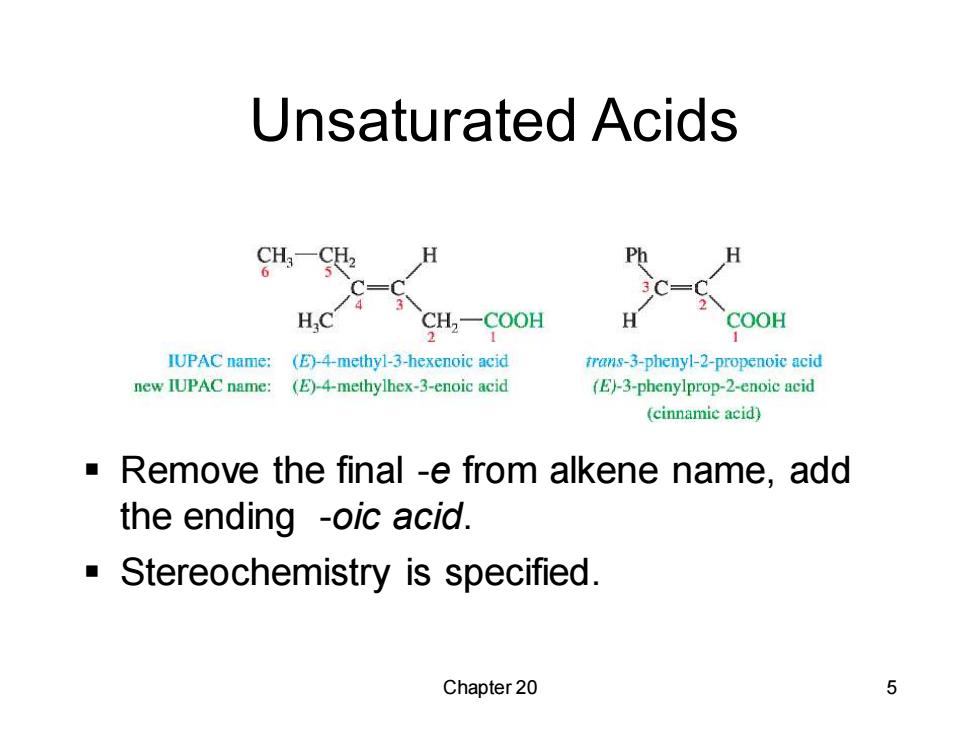
Unsaturated Acids Ph SH,一C0OH H COOH IUPAC name: (E)-4-methy1-3-hexenoic acid trans-3-phenyl-2-propenoic acid new IUPAC name: (E)-4-methylhex-3-enoic acid (E)-3-phenylprop-2-enoic acid (cinnamic acid) Remove the final -e from alkene name,add the ending -oic acid. Stereochemistry is specified. Chapter 20 5
Chapter 20 5 Unsaturated Acids ▪ Remove the final -e from alkene name, add the ending -oic acid. ▪ Stereochemistry is specified