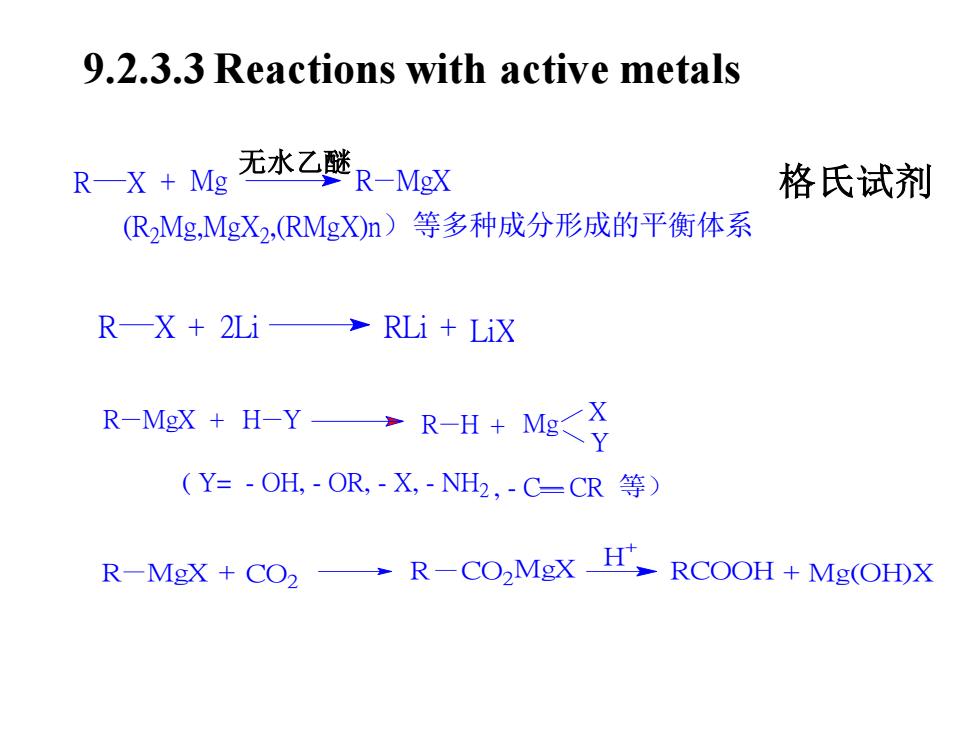
9.2.3.3 Reactions with active metals无水乙醚R-MgX格氏试剂RX + Mg(R2Mg,MgX2,(RMgX)n)等多种成分形成的平衡体系R—X + 2Li→ RLi + LiX R-H + Mg<XR-MgX + H-YY(Y= - OH, - OR, -X,-NH2, - C-CR 等)→ R-CO,MgX H- RCOOH + Mg(OH)XR-MgX + CO2
R X + 2Li RLi + LiX R MgX H Y + Mg X Y + R H ( Y= - OH, - OR, - X, - NH2 , - C CR 等) R M + CO2 gX R CO2 MgX RCOOH + Mg(OH)X H 9.2.3.3 Reactions with active metals R X + Mg R MgX (R2 Mg,MgX2 ,(RMgX)n)等多种成分形成的平衡体系 无水乙醚 格氏试剂
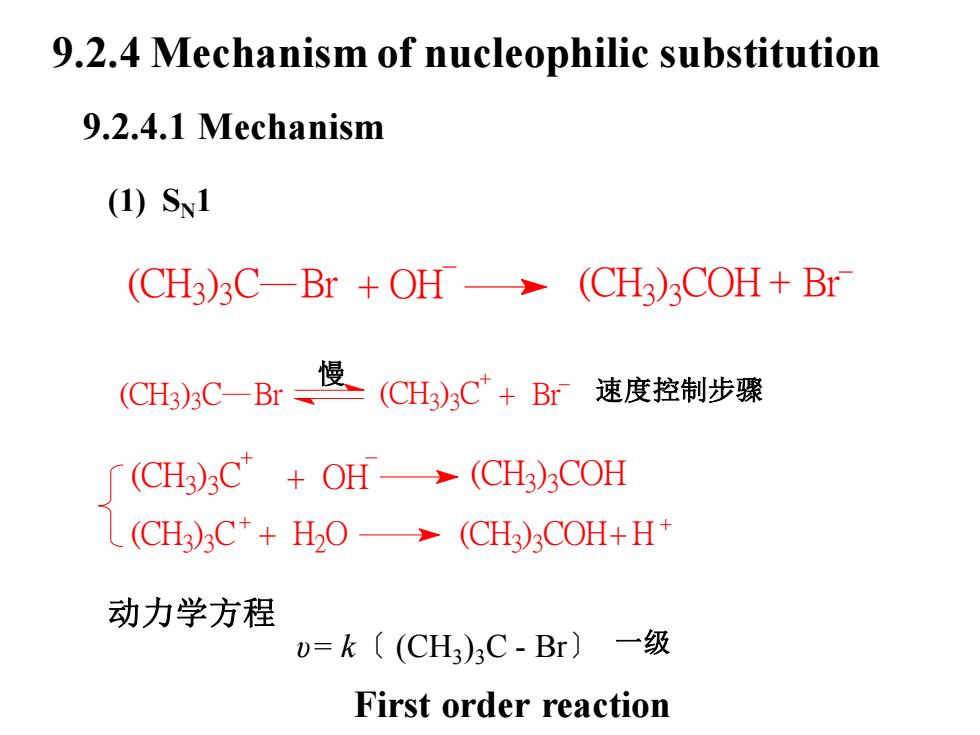
9.2.4 Mechanism of nucleophilic substitution9.2.4.1 Mechanism(1) S1(CH3)3C—Br + OH → (CH3)3COH+ Br慢(CH3);Ct+ Br速度控制步骤(CH3)3C—Br =[ (CH3)3C++ OH→(CH3)COHL(CH3)3C+ + H2O → (CH3)3COH+H+动力学方程U=k((CH3)C-Br)一级First order reaction
(CH3 ) (CH3 ) 3 C + OH 3 COH (CH3 ) 3 C + H2 O (CH3 ) 3 COH+H 动力学方程 υ= k〔 (CH3 )3C - Br〕 一级 9.2.4 Mechanism of nucleophilic substitution 9.2.4.1 Mechanism (1) SN1 (CH3 ) 3 C Br + OH (CH3 ) 3 COH + Br (CH3 ) 3 C Br (CH3 ) 3 C + Br 慢 速度控制步骤 First order reaction