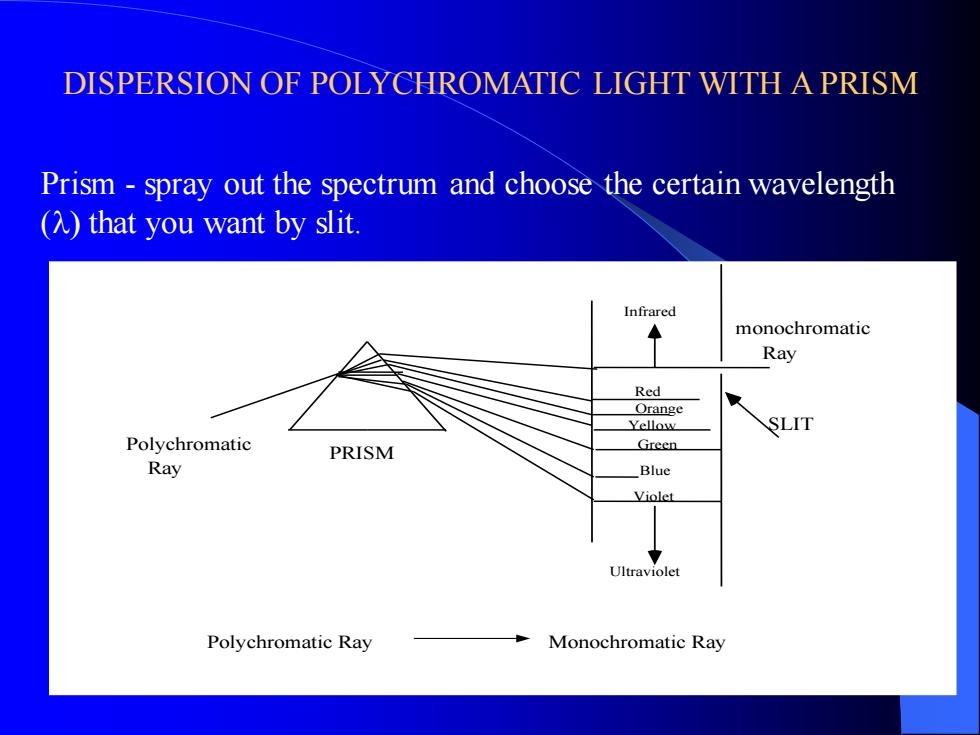
DISPERSION OF POLYCHROMATIC LIGHT WITH A PRISM Polychromatic Ray Infrared Red Orange Yellow Green Blue Violet Ultraviolet monochromatic Ray SLIT PRISM Polychromatic Ray Monochromatic Ray Prism - spray out the spectrum and choose the certain wavelength (l) that you want by slit
DISPERSION OF POLYCHROMATIC LIGHT WITH A PRISM Polychromatic Ray Infrared Red Orange Yellow Green Blue Violet Ultraviolet monochromatic Ray SLIT PRISM Polychromatic Ray Monochromatic Ray Prism - spray out the spectrum and choose the certain wavelength (l) that you want by slit
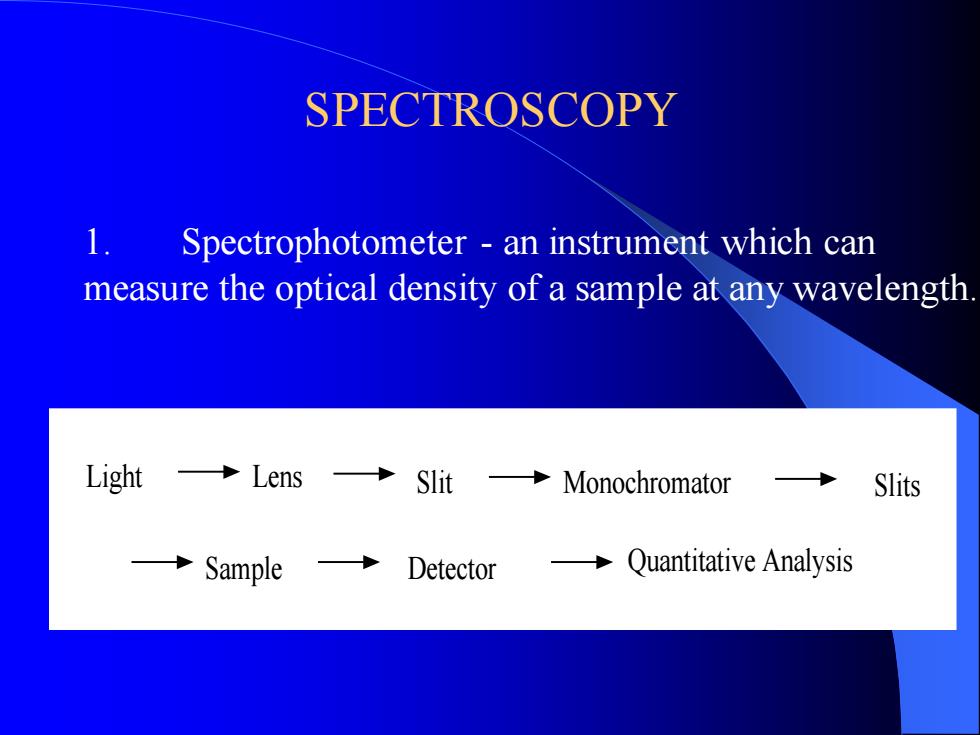
SPECTROSCOPY 1. Spectrophotometer - an instrument which can measure the optical density of a sample at any wavelength. Light Lens Slit Monochromator Sample Detector Quantitative Analysis Slits
SPECTROSCOPY 1. Spectrophotometer - an instrument which can measure the optical density of a sample at any wavelength. Light Lens Slit Monochromator Sample Detector Quantitative Analysis Slits
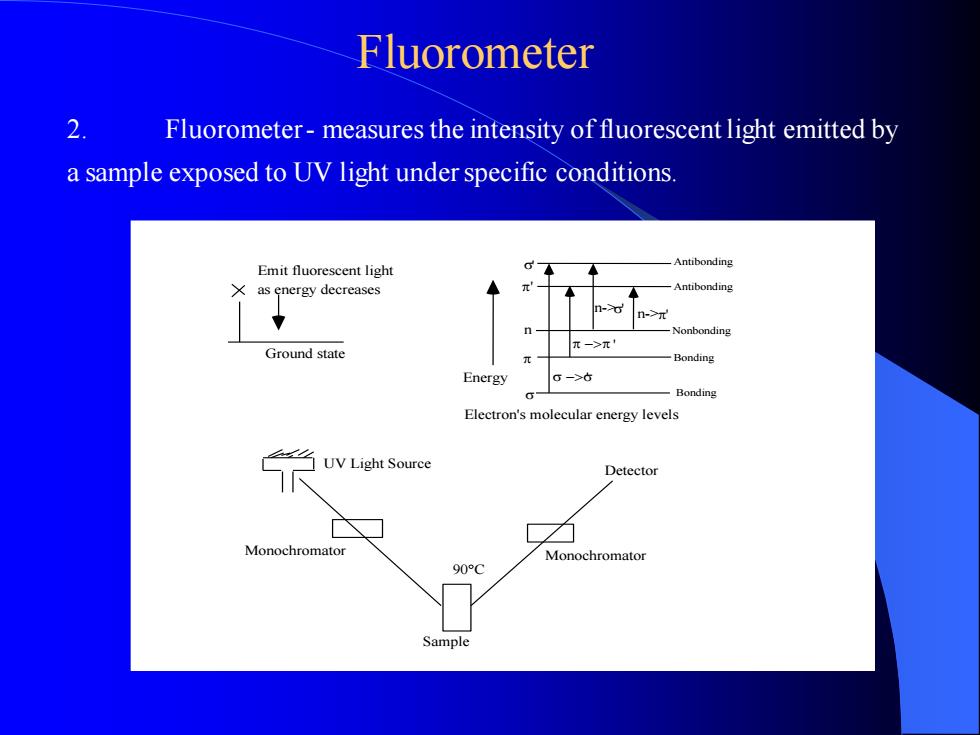
2. Fluorometer - measures the intensity of fluorescent light emitted by a sample exposed to UV light under specific conditions. Emit fluorescent light as energy decreases Ground state Sample 90C Detector UV Light Source Monochromator Monochromator Antibonding Antibonding Nonbonding Bonding Bonding Energy − − ' ' ' ' ' n-> n n->' Electron's molecular energy levels Fluorometer
2. Fluorometer - measures the intensity of fluorescent light emitted by a sample exposed to UV light under specific conditions. Emit fluorescent light as energy decreases Ground state Sample 90C Detector UV Light Source Monochromator Monochromator Antibonding Antibonding Nonbonding Bonding Bonding Energy − − ' ' ' ' ' n-> n n->' Electron's molecular energy levels Fluorometer
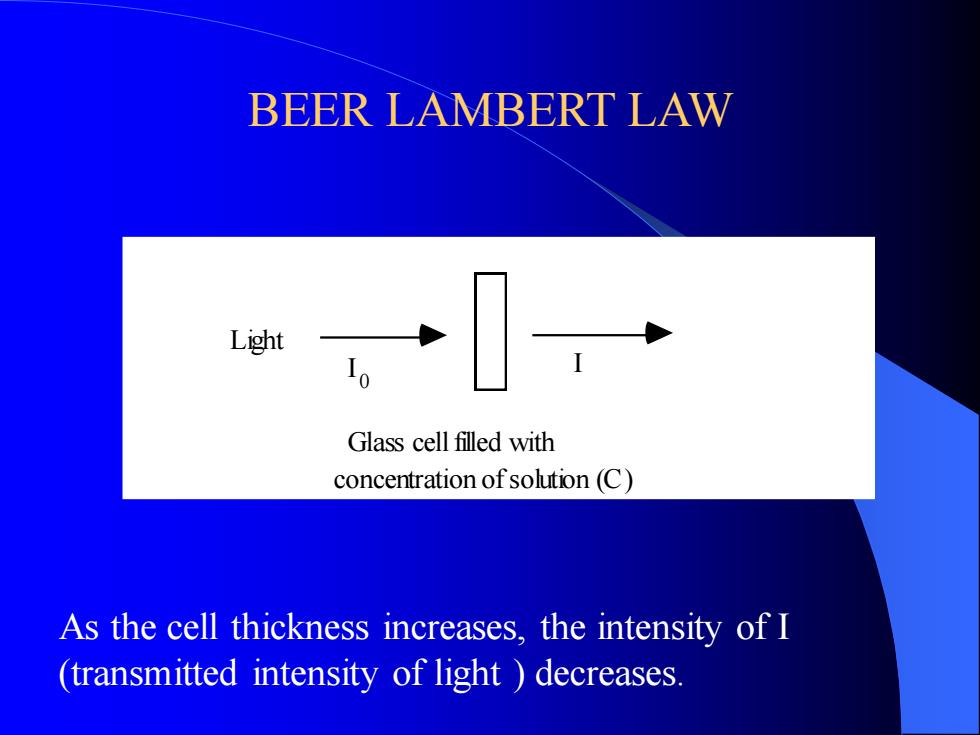
BEER LAMBERT LAW Glass cell filled with concentration of solution (C) I I Light 0 As the cell thickness increases, the intensity of I (transmitted intensity of light ) decreases
BEER LAMBERT LAW Glass cell filled with concentration of solution (C) I I Light 0 As the cell thickness increases, the intensity of I (transmitted intensity of light ) decreases
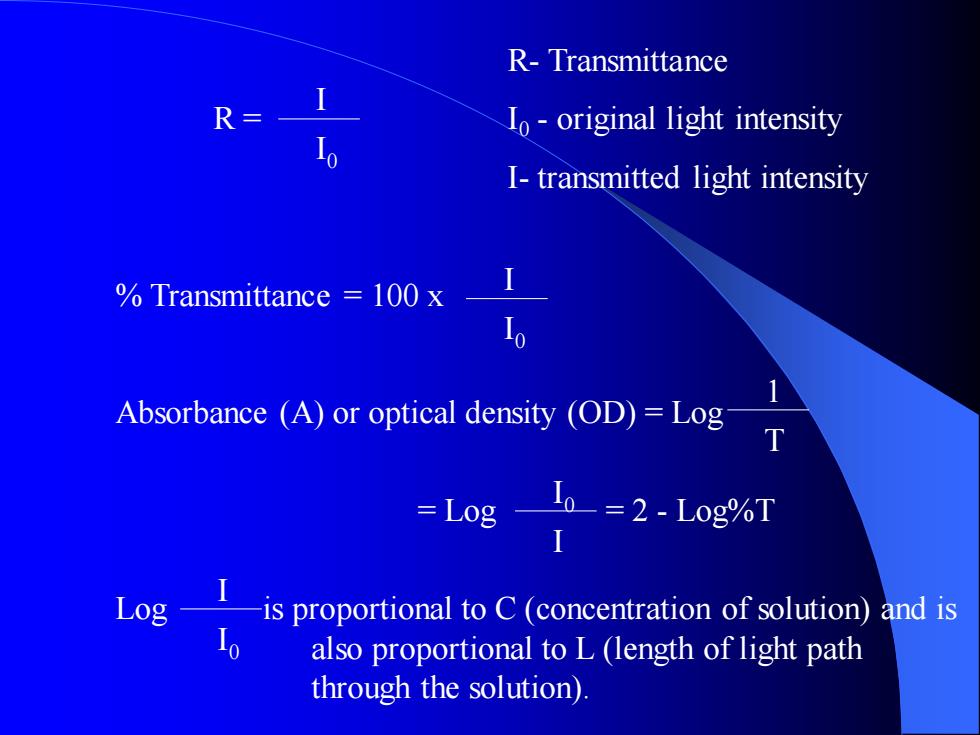
R- Transmittance R = I0 - original light intensity I- transmitted light intensity % Transmittance = 100 x Absorbance (A) or optical density (OD) = Log = Log = 2 - Log%T Log is proportional to C (concentration of solution) and is also proportional to L (length of light path through the solution). I I 0 I I 0 I 0 I 1 T I I 0
R- Transmittance R = I0 - original light intensity I- transmitted light intensity % Transmittance = 100 x Absorbance (A) or optical density (OD) = Log = Log = 2 - Log%T Log is proportional to C (concentration of solution) and is also proportional to L (length of light path through the solution). I I 0 I I 0 I 0 I 1 T I I 0