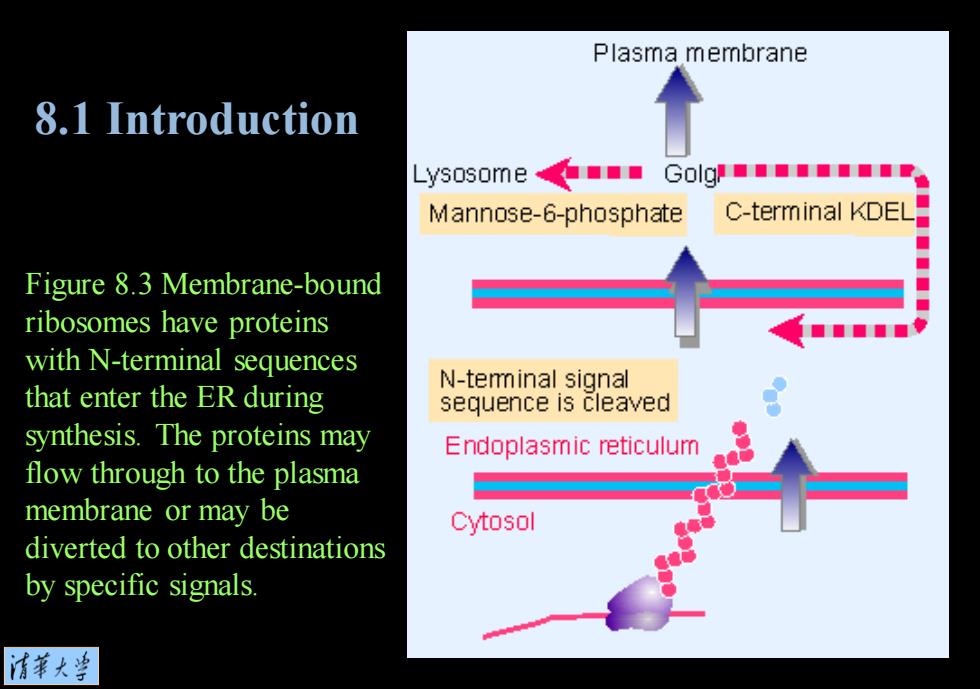
Plasma membrane 8.1 Introduction Lysosome■Golgh" Mannose-6-phosphate C-terminal KDEL Figure 8.3 Membrane-bound ribosomes have proteins ■■■ with N-terminal sequences that enter the ER during N-teminal signal sequence is cleaved synthesis.The proteins may Endoplasmic reticulum flow through to the plasma membrane or may be Cytosol diverted to other destinations by specific signals. 清苇大当
Figure 8.3 Membrane-bound ribosomes have proteins with N-terminal sequences that enter the ER during synthesis. The proteins may flow through to the plasma membrane or may be diverted to other destinations by specific signals. 8.1 Introduction

8.2 Chaperones may be required Protein acquires confom ation after for protein folding membrane passage 通 Protein must pass through channel in m emhrane Figure 8.4 A protein is constrained to a Folded confom ation narrow passage as it could prevent passage through membrane crosses a membrane. 情華大当
Figure 8.4 A protein is constrained to a narrow passage as it crosses a membrane. 8.2 Chaperones may be required for protein folding

8.2 Chaperones may be required for protein folding System Function Hsp70 Hsp70 (DnaK) ATPase Hsp40 (DnaJ) stimulates ATP ase GrpE (GrpE) Nucleotide exchange factor Chaperonin Hsp60 (GroEL) Foms two heptameric rings; Hsp10 (GroES) Fomms cap Figure 8.5 Chaperone families have eukaryotic and bacterial counterparts (named in parentheses). 清第大当
Figure 8.5 Chaperone families have eukaryotic and bacterial counterparts (named in parentheses). 8.2 Chaperones may be required for protein folding
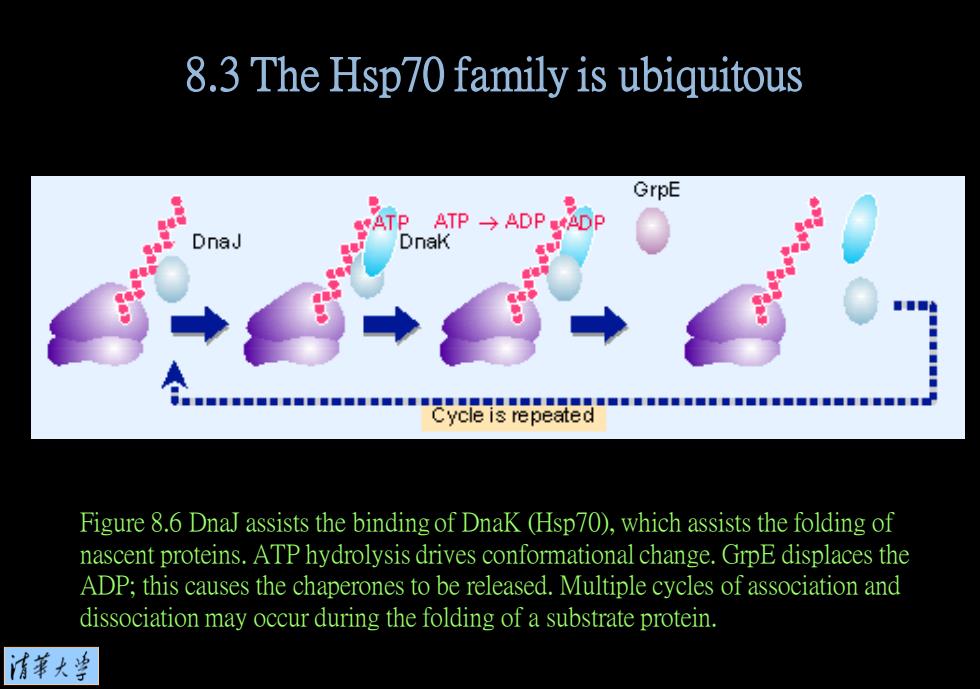
8.3 The Hsp70 family is ubiquitous GrpE ATPATP→ADP.AF DnaJ DnaK Figure 8.6 DnaJ assists the binding of DnaK (Hsp70),which assists the folding of nascent proteins.ATP hydrolysis drives conformational change.GrpE displaces the ADP;this causes the chaperones to be released.Multiple cycles of association and dissociation may occur during the folding of a substrate protein. 清菜大当
Figure 8.6 DnaJ assists the binding of DnaK (Hsp70), which assists the folding of nascent proteins. ATP hydrolysis drives conformational change. GrpE displaces the ADP; this causes the chaperones to be released. Multiple cycles of association and dissociation may occur during the folding of a substrate protein. 8.3 The Hsp70 family is ubiquitous

8.4 Hsp60/GroEL Pr otein enters throu gh forms an en d of cylinder oligomeric ring structure Figure 8.7-1 A protein may be sequestered within a controlled environment for folding or Protein interacts only degradation. with walls of cavity 清苇大兰
Figure 8.7-1 A protein may be sequestered within a controlled environment for folding or degradation. 8.4 Hsp60/GroEL forms an oligomeric ring structure