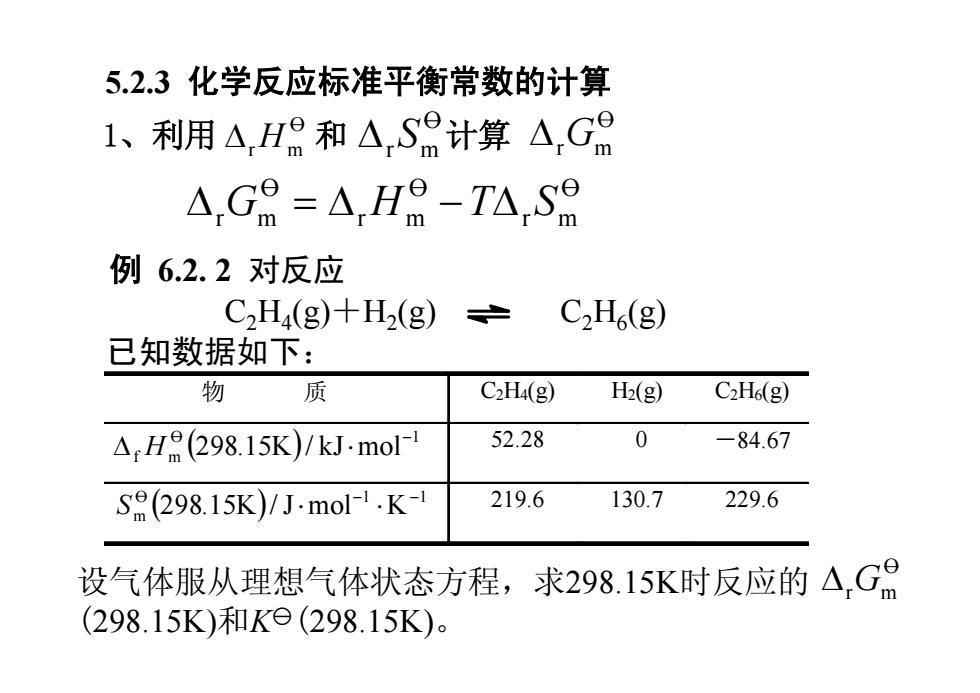
5.2.3化学反应标准平衡常数的计算 1、利用A,H8和△,S8计算△,G日 △,G8=△,Ha-TA,S9 例6.2.2对反应 C2H4(g)+H2(g) C2H(g) 已知数据如下: 物 质 C2H4(g) H2(g) C2H6(g) ,H(298.15K)/kJ.mol- 52.28 0 -84.67 Sa(298.15K)/Jmol-1.K- 219.6 130.7 229.6 设气体服从理想气体状态方程,求298.15K时反应的△,G品 (298.15K)和Ke(298.15K)
5.2.3 化学反应标准平衡常数的计算 1、利用 和 计算 O DrGm O r m O r m O r m D G = D H -TD S O DrH m O DrSm 例 6.2. 2 对反应 已知数据如下: C2H4 (g)+H2 (g) C2H6 (g) 物 质 C2H4(g) H2(g) C2H6(g) ( ) O 1 f m 298.15K / kJ mol- D H × 52.28 0 -84.67 ( ) O 1 1 m 298.15K / J mol K - - S × × 219.6 130.7 229.6 设气体服从理想气体状态方程,求298.15K时反应的 (298.15K)和Ky(298.15K)。 O DrGm

解:△,H(298K)=∑aYgA,H(B,298.15K) =(-84.67-52.28+0)kJmol-1 =-136.95 kJ-mol-1 △,SR(298.15K)=∑BVgS8B,298.15K) =(229.6-219.6-130.7)Jmol-1K-1 =-120.7Jmol-1K-1 △,GR(298.15K)=A,HR(298.15K)-T△,S(298.15K) =-136.95 kJ-mol-1-298.15K×(-120.7×10-3 kJmol-1.K-1) =-100.96kJmo1 K2815K)-er- G(298.15K) RT -exp- -100.96×103 =4.88×1017 .314×298.15
解: (298 ) (B,298.15K) O B B f m O DrHm K = å n D H =(-84.67-52.28+0)kJ·mol-1 =-136.95kJ·mol-1 (298.15K) (B,298.15K) O B B m O DrSm = å n S =(229.6-219.6-130.7) J·mol-1 ·K-1 =-120.7 J·mol-1 ·K-1 (298.15K) (298.15K) (298.15K) O r m O r m O DrGm =D H -TD S =-136.95kJ·mol-1-298.15K×(-120.7 ×10-3 kJ·mol-1·K-1) =-100.96 kJ·mol-1 ( ) ( ) 17 3 O O r m 4.88 10 8.314 298.15 100.96 10 exp 298.15K 298.15K exp = ´ ÷ ÷ ø ö ç ç è æ ´ - ´ = - ÷ ÷ ø ö ç ç è æ D - RT G K =