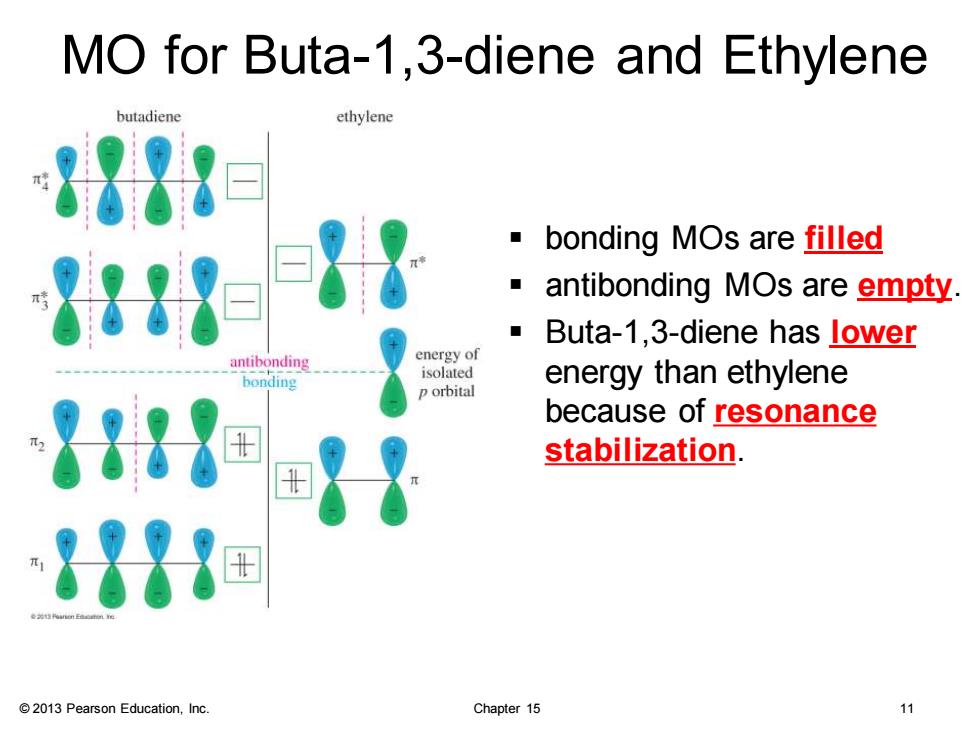
MO for Buta-1,3-diene and Ethylene butadiene ethylene 1= bonding MOs are filled antibonding MOs are empty Buta-1,3-diene has lower energy of bonding isolated energy than ethylene p orbital 8▣} because of resonance stabilization. 821田 2013 Pearson Education,Inc. Chapter 15 11
© 2013 Pearson Education, Inc. Chapter 15 11 MO for Buta-1,3-diene and Ethylene ▪ bonding MOs are filled ▪ antibonding MOs are empty. ▪ Buta-1,3-diene has lower energy than ethylene because of resonance stabilization

Conformations of Buta-1,3-diene The s-trans conformer is more stable than the s-cis by 12 kJ/mol (2.8 kcal/mol). Easily interconvert at room temperature. H mild 2 interference H H s-trans S-cis 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 12 Conformations of Buta-1,3-diene ▪ The s-trans conformer is more stable than the s-cis by 12 kJ/mol (2.8 kcal/mol). ▪ Easily interconvert at room temperature
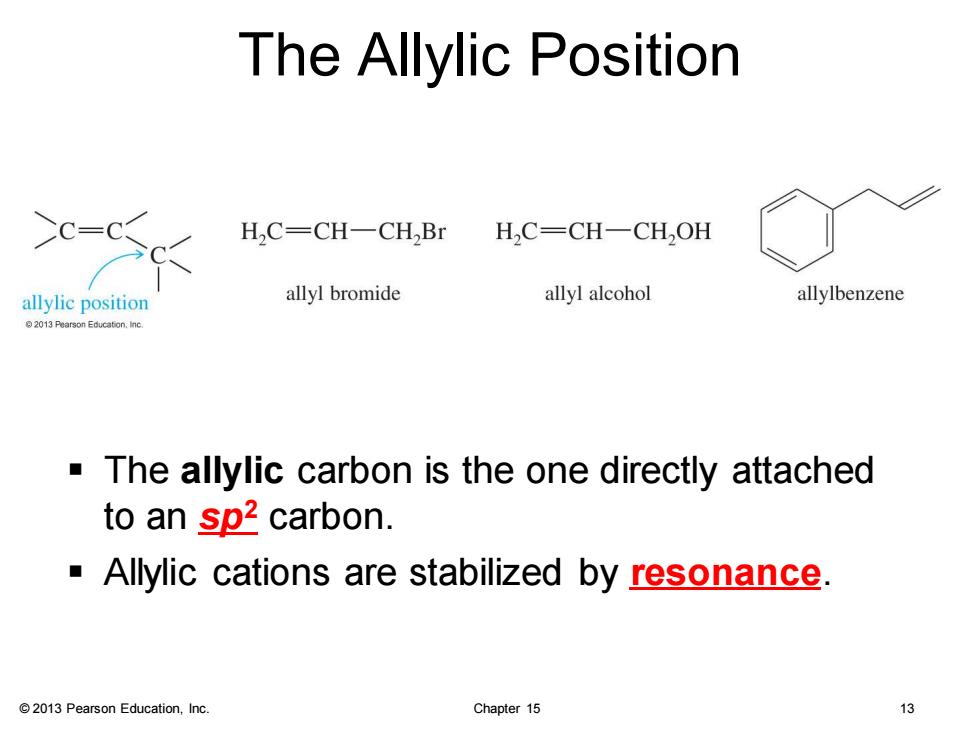
The Allylic Position H,C-CH-CH,Br HC=CH-CH,OH allylic position allyl bromide allyl alcohol allylbenzene 2013 Pearson Education.Inc. The allylic carbon is the one directly attached to an sp2 carbon. Allylic cations are stabilized by resonance. 2013 Pearson Education,Inc. Chapter 15 13
© 2013 Pearson Education, Inc. Chapter 15 13 The Allylic Position ▪ The allylic carbon is the one directly attached to an sp2 carbon. ▪ Allylic cations are stabilized by resonance

Allylic Cations H.C-CH-CH,Br: H,C=CH-CH,←→H,C-CH=CH+:Br: allyl bromide allyl cation H H C H H,C=CH一CH-CH C=CH-CH2 CH3 H H substituted allylic cations The positive charge is delocalized over two carbons by resonance the allyl cation is more stable than nonconjugated cations. 2013 Pearson Education,Inc. Chapter 15 14
© 2013 Pearson Education, Inc. Chapter 15 14 Allylic Cations ▪ The positive charge is delocalized over two carbons by resonance ▪ the allyl cation is more stable than nonconjugated cations

Stability of Carbocations HCt<1°<2°,allyl<3°,substituted allylic H,C-CH--CH is about as stable as CH:-CH-CH ·Stability of1 allylic≈2°carbocation. "Stability of2°allylic≈3 carbocation. 2013 Pearson Education,Inc. Chapter 15 15
© 2013 Pearson Education, Inc. Chapter 15 15 Stability of Carbocations ▪ Stability of 1 allylic 2 carbocation. ▪ Stability of 2 allylic 3 carbocation