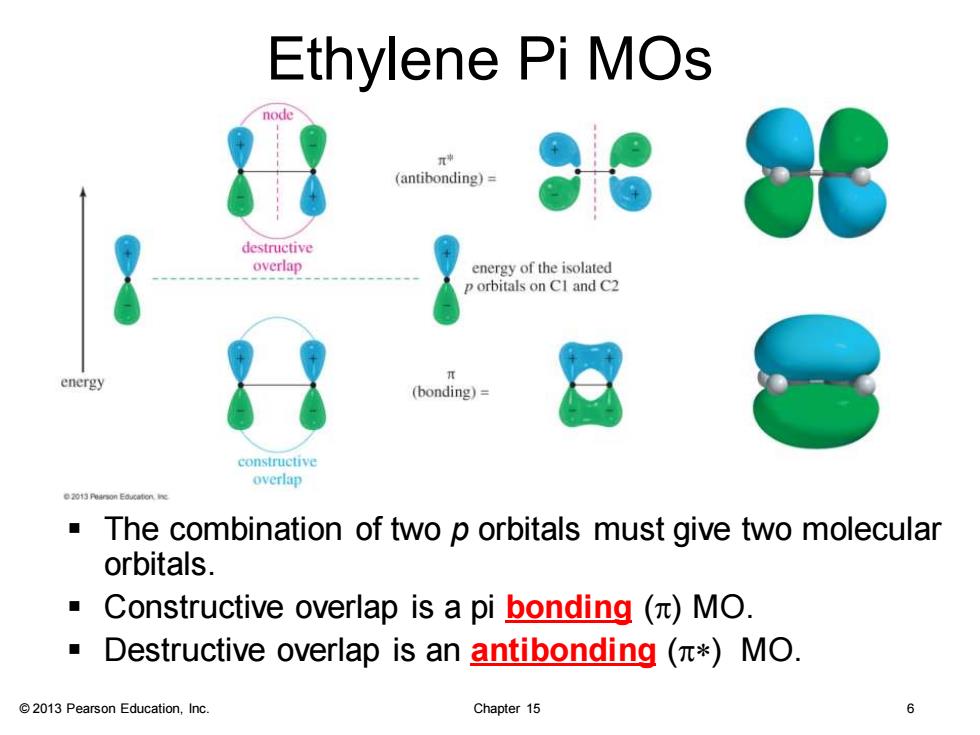
Ethylene Pi MOs node (antibonding)= destructive overlap energy of the isolated orbitals on CI and C2 energy (bonding)= constructive overlap The combination of two p orbitals must give two molecular orbitals. ■ Constructive overlap is a pi bonding()MO. Destructive overlap is an antibonding (*MO. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 6 Ethylene Pi MOs ▪ The combination of two p orbitals must give two molecular orbitals. ▪ Constructive overlap is a pi bonding (p) MO. ▪ Destructive overlap is an antibonding (p*) MO
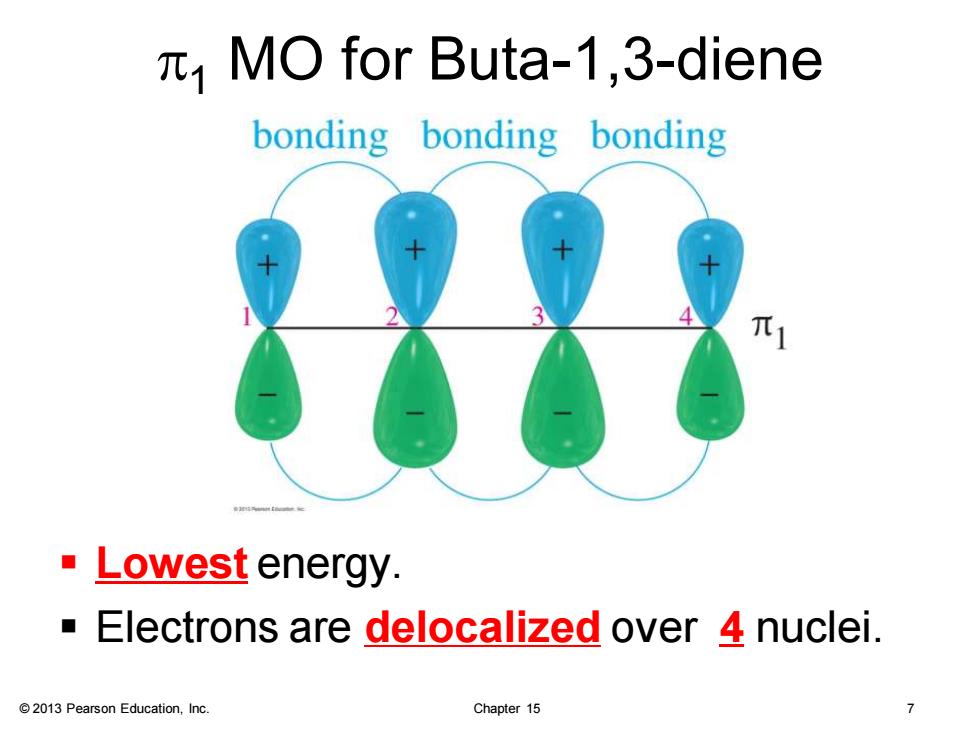
MO for Buta-1,3-diene bonding bonding bonding ·Lowest energy. Electrons are delocalized over 4 nuclei. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 7 p1 MO for Buta-1,3-diene ▪ Lowest energy. ▪ Electrons are delocalized over 4 nuclei
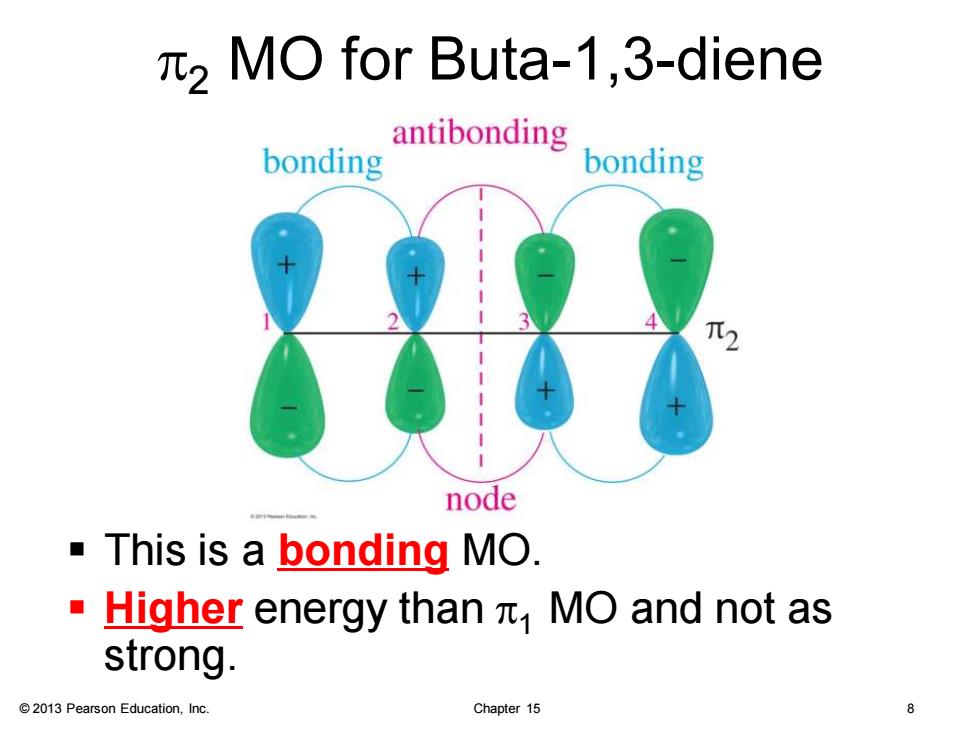
2 MO for Buta-1,3-diene bonding antibonding bonding node This is a bonding MO. Higher energy than MO and not as strong. 2013 Pearson Education,Inc. Chapter 15
© 2013 Pearson Education, Inc. Chapter 15 8 p2 MO for Buta-1,3-diene ▪ This is a bonding MO. ▪ Higher energy than p1 MO and not as strong
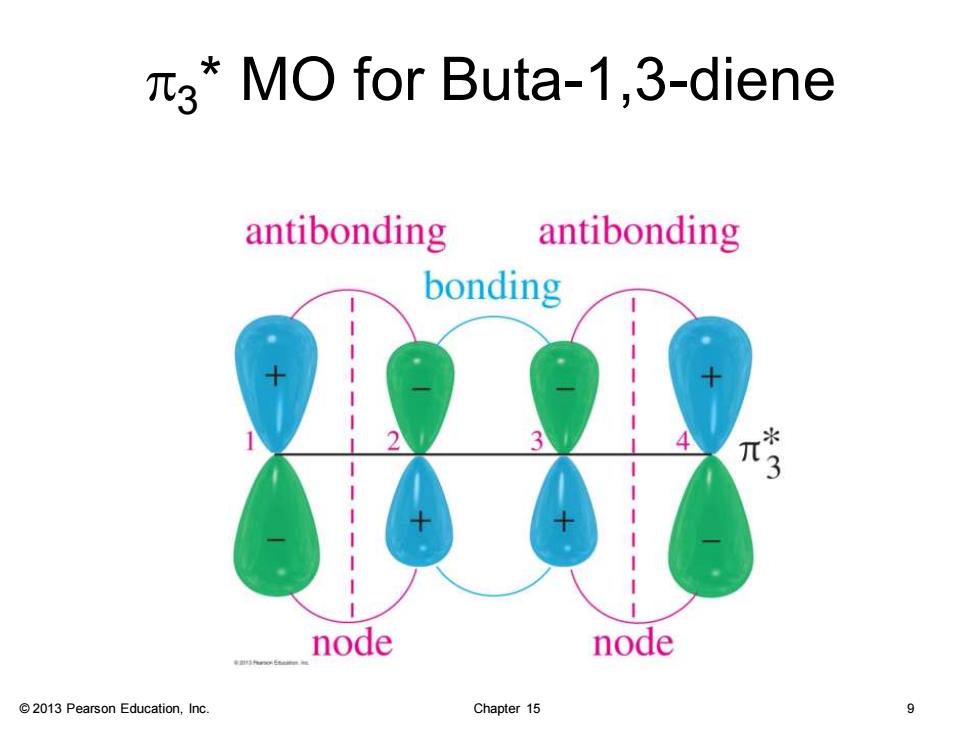
3*MO for Buta-1,3-diene antibonding antibonding bonding 3 node node 2013 Pearson Education,Inc. Chapter 15 9
© 2013 Pearson Education, Inc. Chapter 15 9 p3 * MO for Buta-1,3-diene
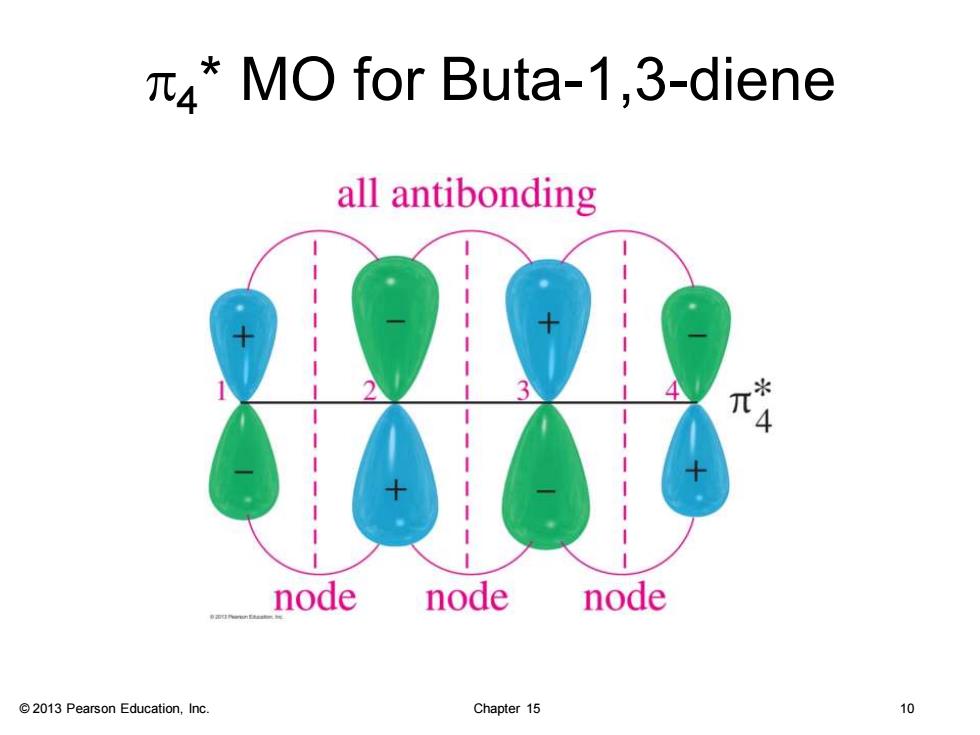
MO for Buta-1,3-diene all antibonding 4 node node node 2013 Pearson Education,Inc. Chapter 15 10
© 2013 Pearson Education, Inc. Chapter 15 10 p4 * MO for Buta-1,3-diene