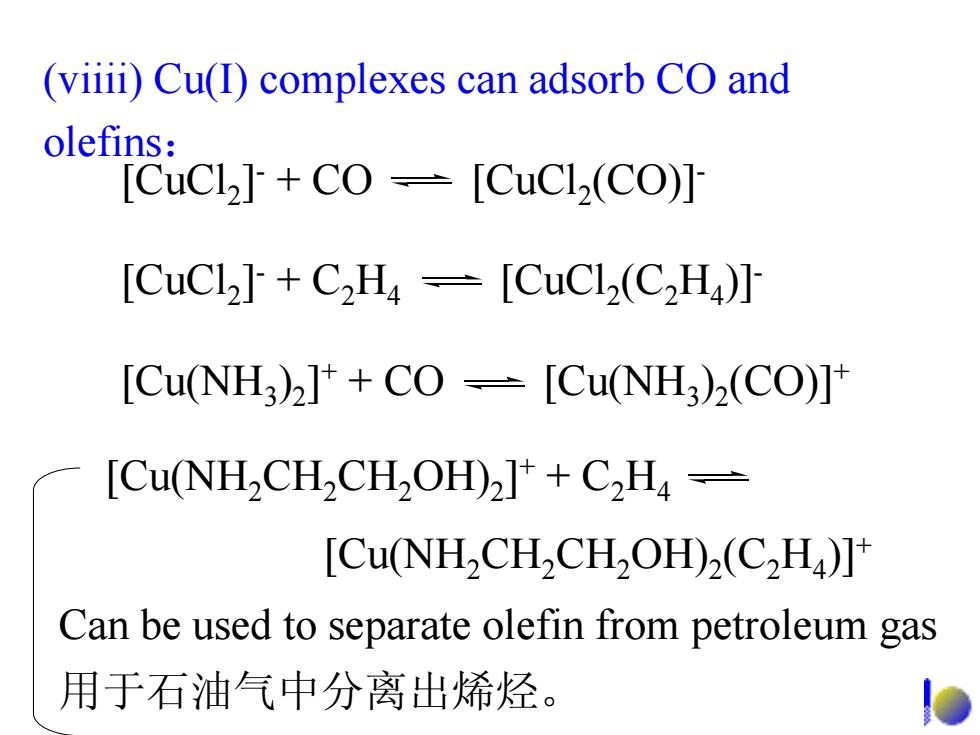
(viiii)Cu(I)complexes can adsorb CO and olefins: [CuCl2]+CO [CuCl2(CO)] [CuCl2]+C2H-[CuCl2(C2H)] [Cu(NH3)2]++CO-[Cu(NH3)2(CO)] [Cu(NH2CH2CH2OH)2]+C2H- [Cu(NH2CH2CH2OH)2(CH)] Can be used to separate olefin from petroleum gas 用于石油气中分离出烯烃
(viiii) Cu(I) complexes can adsorb CO and olefins : [CuCl 2 ] - + CO [CuCl 2(CO)] - [CuCl 2 ] - + C 2 H 4 [CuCl 2(C 2 H 4)] - [Cu(NH 3 ) 2 ] + + CO [Cu(NH 3 ) 2(CO)] + [Cu(NH 2CH 2CH 2OH) 2 ] + + C 2 H 4 [Cu(NH 2CH 2CH 2OH) 2(C 2 H 4)] + Can be used to separate olefin from petroleum gas 用于石油气中分离出烯烃
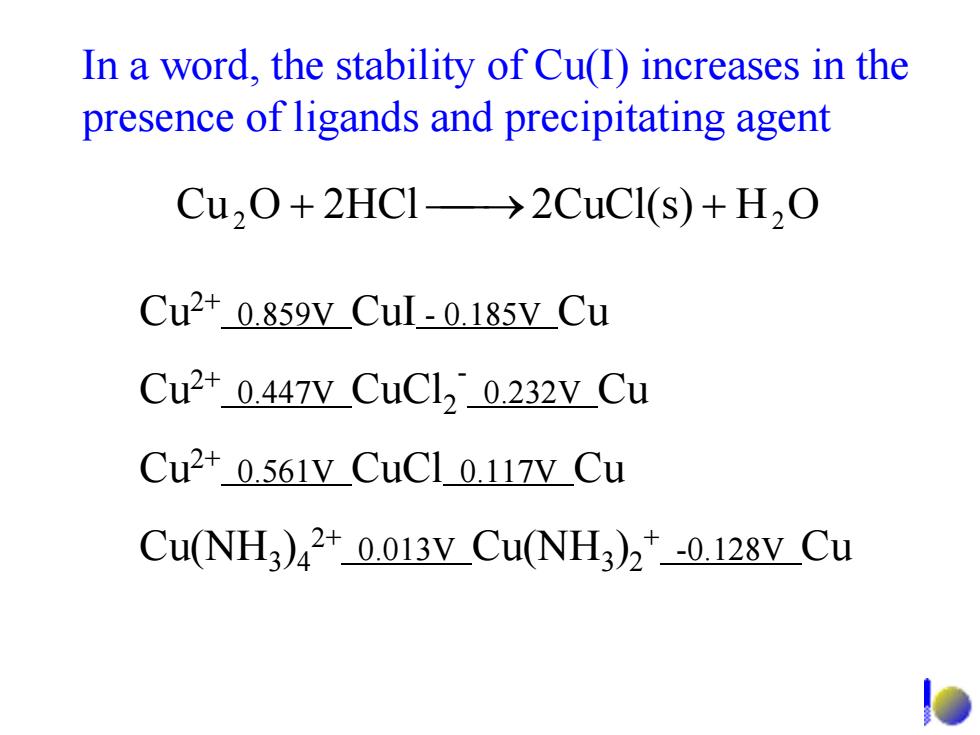
In a word,the stability of Cu(I)increases in the presence of ligands and precipitating agent Cu,O+2HCI->2CuCl(s)+H,O Cu2+0.859v Cul-0.185V Cu Cu2+0.447V CuCl,0.232V_Cu Cu2+0.561V_CuC10.117V_Cu Cu(NH3)42+0.013 v_Cu(NH3)2+-0.128VCu
Cu2+ 0.859V CuI - 0.185V Cu Cu2+ 0.447V CuCl2- 0.232V Cu Cu2+ 0.561V CuCl 0.117V Cu Cu(NH3)42+ 0.013V Cu(NH3)2+ -0.128V Cu 2 2HClOCu ⎯+ ⎯→2CuCl(s) + 2OH In a word, the stability of Cu(I) increases in the presence of ligands and precipitating agent