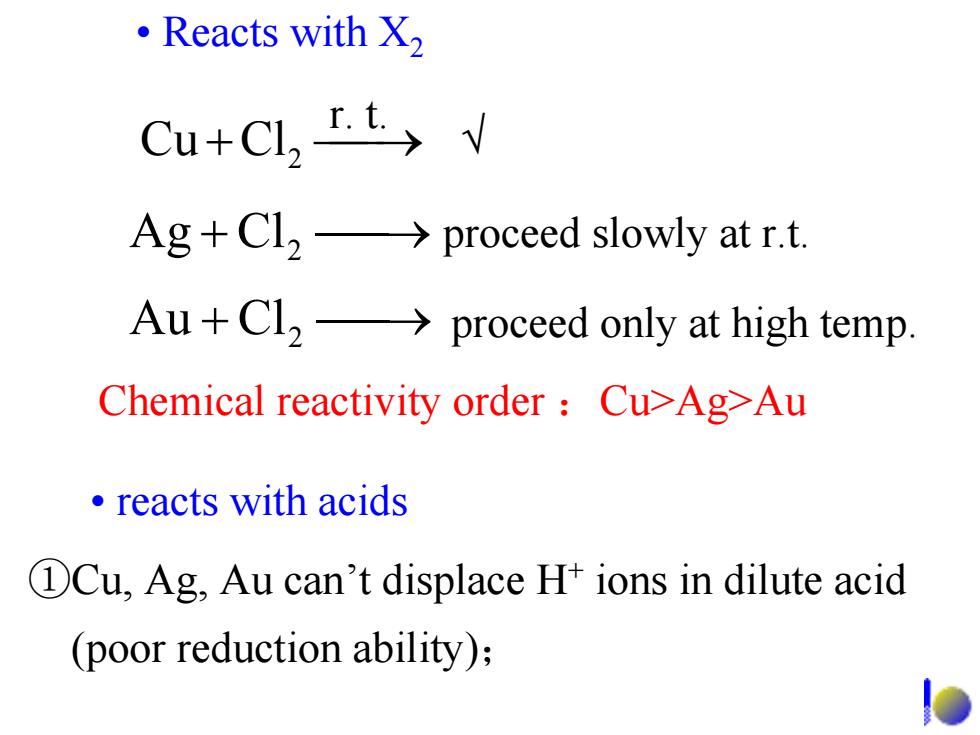
·Reacts with X) Cu+Cl,I.t> Ag+Cl2->proceed slowly at r.t. Au+Cl2->proceed only at high temp. Chemical reactivity order Cu>Ag-Au ·reacts with acids DCu,Ag,Au can't displace H+ions in dilute acid (poor reduction ability);
• Reacts with X2 ClCu 2 ⎯+ ⎯→r. t. Chemical reactivity order :Cu>Ag>Au ⎯+ ⎯→ proceed only at high temp. ClAu 2 ⎯+ ⎯→ proceed slowly at r.t. ClAg 2 • reacts with acids ①Cu, Ag, Au can’t displace H+ ions in dilute acid (poor reduction ability); √
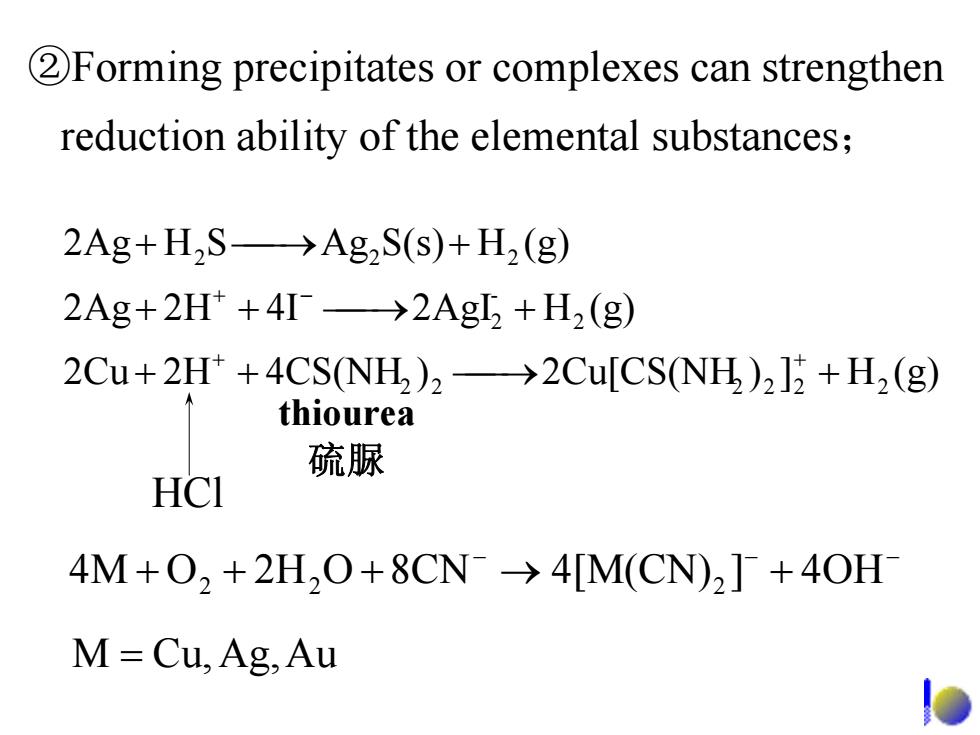
2Forming precipitates or complexes can strengthen reduction ability of the elemental substances; 2Ag+H2S->Ag2S(s)+H2(g) 2Ag+2H+4I→2Ag+H2(g) 2Cu+2H*+4CS(NH)2->2Cu[CS(NH)212 +H2(g) thiourea 硫脲 HCI 4M+0,+2H,O+8CN->4[M(CN),+40H M=Cu,Ag,Au
2H2Cu )4CS(NH 2Cu[CS(NH (g)H]) 4I2H2Ag (g)H2AgI (g)HS(s)AgSH2Ag 22 2222 2 - 2 2 2 2 ++ ⎯⎯→ + ⎯++ ⎯→ + ⎯+ ⎯→ + + + −+ HCl thiourea 硫脲 ②Forming precipitates or complexes can strengthen reduction ability of the elemental substances; − − − 8CNO2HO4M →+++ + 4OH]4[M(CN) 22 2 = AuAg,Cu,M
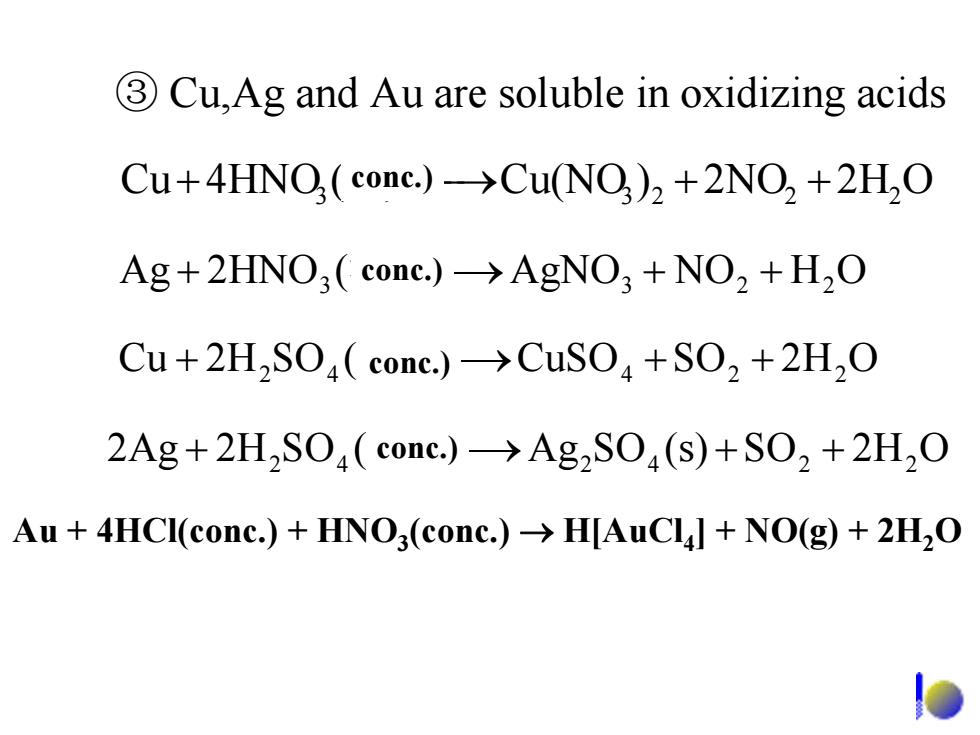
3Cu,Ag and Au are soluble in oxidizing acids Cu+4HNO(cone.)->Cu(NO)2+2NO2 +2H2O Ag+2HNO3 cone.)->AgNO3 +NO2 +H2O Cu+2H2SO(cone.)->CuSO+SO2+2H2O 2Ag+2H2SO(conc.)->Ag2SO(s)+SO2+2H2O Au 4HCI(conc.)+HNO3(conc.)>H[AuCll NO(g)+2H2O
③ Cu,Ag and Au are soluble in oxidizing acids + 3 浓)(4HNOCu ⎯⎯→ 23 ++ 22 O2H2NO)Cu(NO O2HSO(s)SOAg)(SO2H2Ag + 42 浓 ⎯⎯→ 42 ++ 22 + 42 浓)(SO2HCu ⎯⎯→CuSO ++ 224 O2HSO + 3 浓)(2HNOAg ⎯⎯→AgNO3 ++ 22 OHNO conc.) conc.) conc.) conc.) Au + 4HCl(conc.) + HNO3(conc.) → H[AuCl4] + NO(g) + 2H2O

17.1.2 The compounds of copper 1.The compounds of copper Cu(①):dlo,Compounds of Cu(①are white or colorless, no d-d transition Cu(⑩:d',Compounds of Cu(I)have colors, d-d transition Cu(IID):d8,K3CuF(light green),strong oxidizing agent d-d transition
1.The compounds of copper 17.1.2 The compounds of copper Cu(I): d10, Compounds of Cu(I) are white or colorless, no d-d transition Cu(II): d 9, Compounds of Cu(II) have colors, d-d transition Cu(III): d 8, K3CuF 6(light green), strong oxidizing agent d-d transition

17.1.2 The compounds of copper 1.The compounds of copper Cu⑩:do,Compounds of Cu⑩are white or colorless, no d-d transition Cu(ID:d9,Compounds of Cu(II)have colors, d-d transition Cu(IID):d3,K3CuF(light green),strong oxidizing agent d-d transition
1.The compounds of copper 17.1.2 The compounds of copper Cu(I): d10, Compounds of Cu(I) are white or colorless, no d-d transition Cu(II): d 9, Compounds of Cu(II) have colors, d-d transition Cu(III): d 8, K3CuF 6(light green), strong oxidizing agent d-d transition