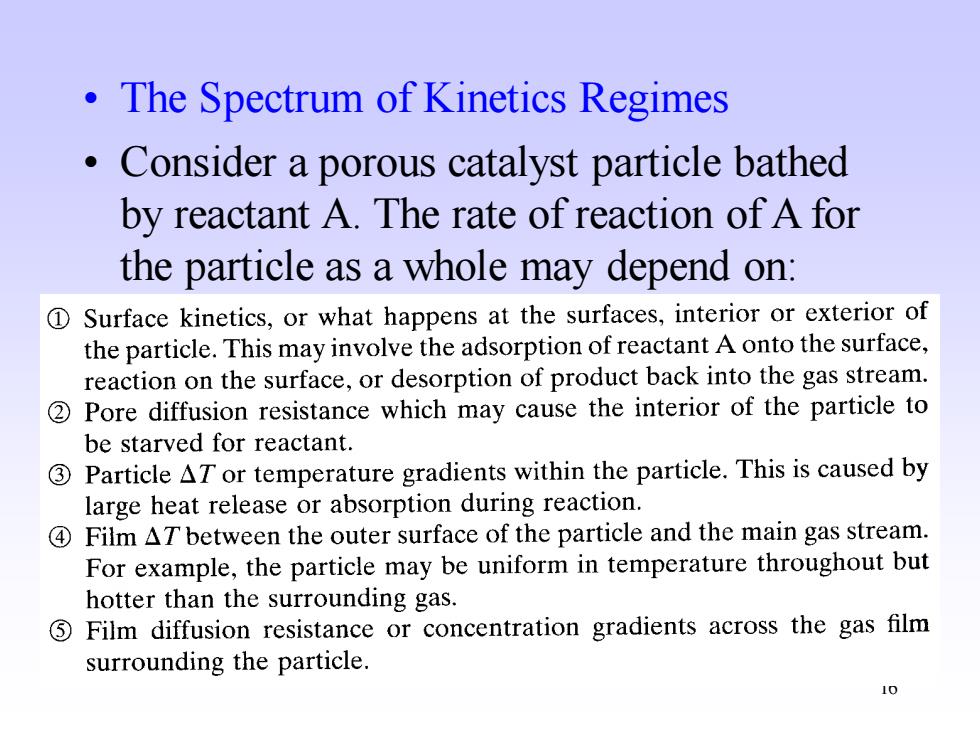
The Spectrum of Kinetics Regimes Consider a porous catalyst particle bathed by reactant A.The rate of reaction of a for the particle as a whole may depend on: 1 Surface kinetics,or what happens at the surfaces,interior or exterior of the particle.This may involve the adsorption of reactant A onto the surface, reaction on the surface,or desorption of product back into the gas stream. 2Pore diffusion resistance which may cause the interior of the particle to be starved for reactant. 3Particle AT or temperature gradients within the particle.This is caused by large heat release or absorption during reaction. 4 Film AT between the outer surface of the particle and the main gas stream. For example,the particle may be uniform in temperature throughout but hotter than the surrounding gas. ⑤ Film diffusion resistance or concentration gradients across the gas film surrounding the particle
16 • The Spectrum of Kinetics Regimes • Consider a porous catalyst particle bathed by reactant A. The rate of reaction of A for the particle as a whole may depend on:
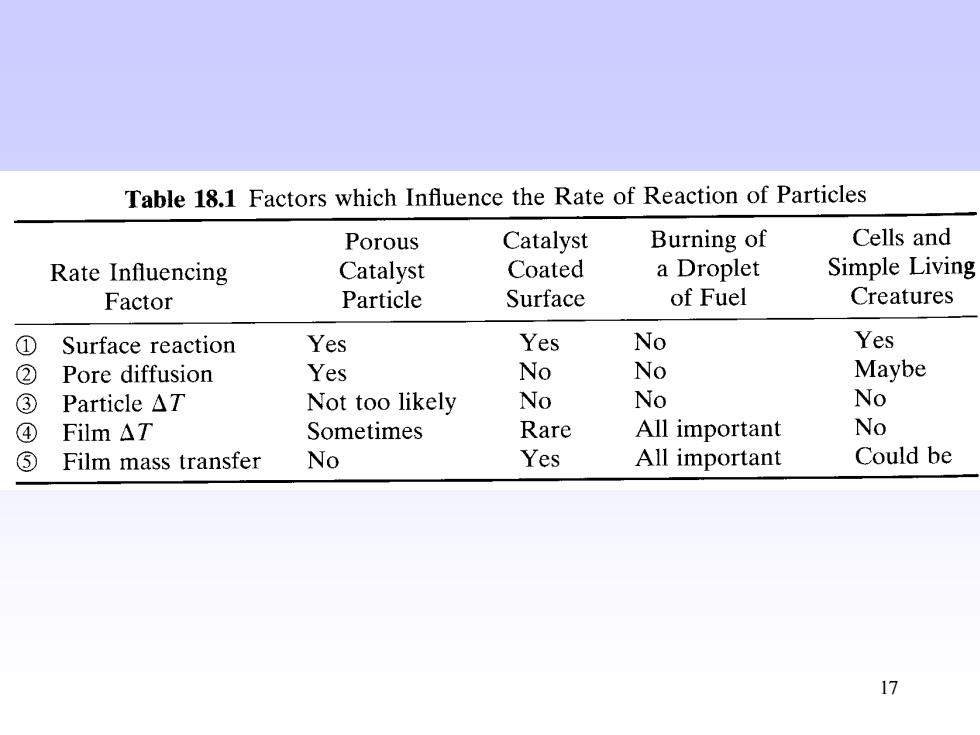
Table 18.1 Factors which Influence the Rate of Reaction of Particles Porous Catalyst Burning of Cells and Rate Influencing Catalyst Coated a Droplet Simple Living Factor Particle Surface of Fuel Creatures ① Surface reaction Yes Yes No Yes ② Pore diffusion Yes No No Maybe ③ Particle△T Not too likely No No No ④ Film△T Sometimes Rare All important No ⑤ Film mass transfer No Yes All important Could be 17
17

18.1 The Rate Equation for Surface Kinetics Because of the great industrial importance of catalytic reactions,considerable effort has been spent in developing theories from which kinetic equations can rationally be developed.The most useful for our purposes supposes that the reaction takes place on an active site on the surface of the catalyst.Thus three steps are viewed to occur successively at the surface. 18
18 18.1 The Rate Equation for Surface Kinetics • Because of the great industrial importance of catalytic reactions, considerable effort has been spent in developing theories from which kinetic equations can rationally be developed. The most useful for our purposes supposes that the reaction takes place on an active site on the surface of the catalyst. Thus three steps are viewed to occur successively at the surface
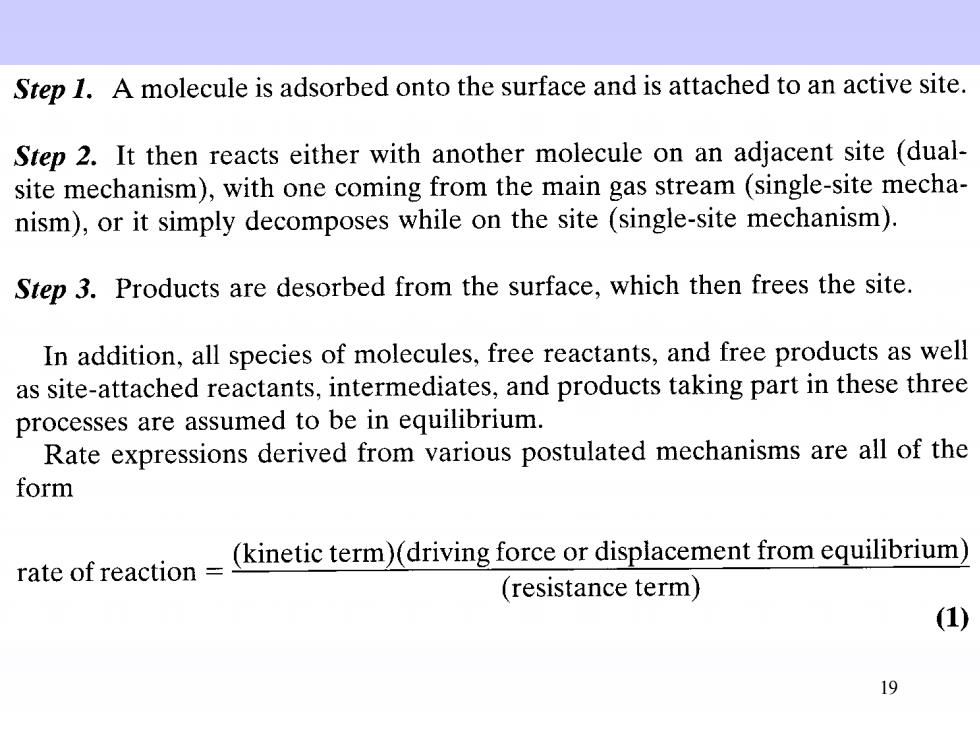
Step 1.A molecule is adsorbed onto the surface and is attached to an active site Step 2.It then reacts either with another molecule on an adjacent site (dual- site mechanism),with one coming from the main gas stream (single-site mecha- nism),or it simply decomposes while on the site(single-site mechanism). Step 3.Products are desorbed from the surface,which then frees the site. In addition,all species of molecules,free reactants,and free products as well as site-attached reactants,intermediates,and products taking part in these three processes are assumed to be in equilibrium. Rate expressions derived from various postulated mechanisms are all of the form rate of reaction-kineti term)(driving force or displacement fromeqm) (resistance term) (1) 19
19
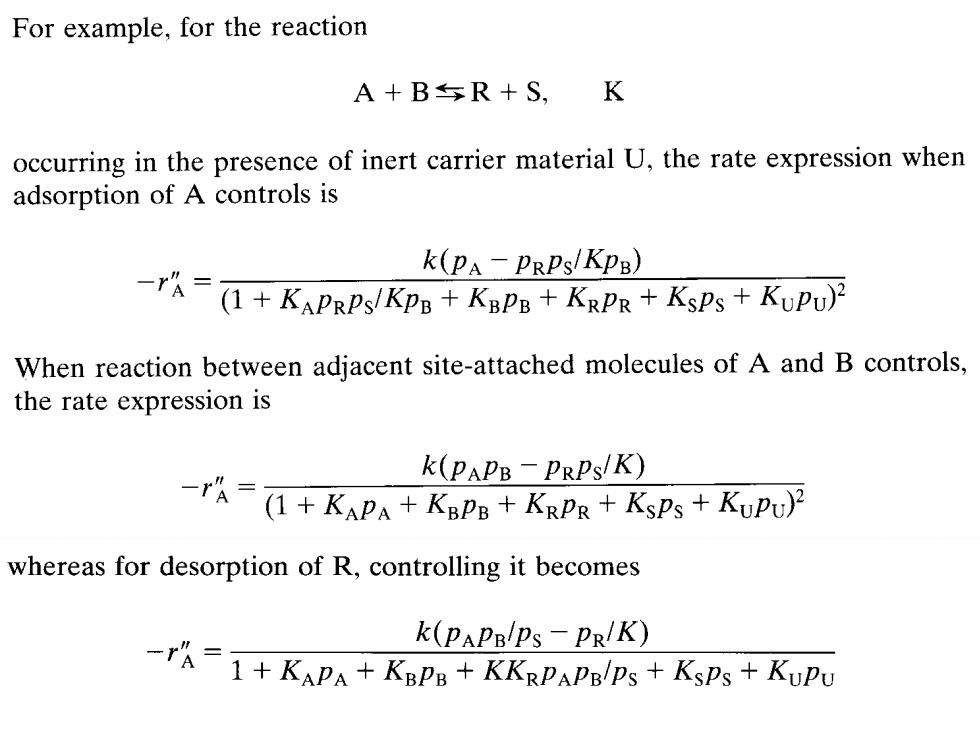
For example,for the reaction A+BR+S,K occurring in the presence of inert carrier material U,the rate expression when adsorption of A controls is k(PA-PRPs/KP8) A-(1+KAPRPs/Kpp+Knp8+KRPR+KsPs KuPu) When reaction between adjacent site-attached molecules of A and B controls, the rate expression is k(PAPB-PRPs/K) 1+KP+Kapp+KaPR+KsPs+KuPu) whereas for desorption of R,controlling it becomes k(PAPBlps-PR/K) 1+KAPA+KaPs+KKaPAPnlps KsPs+KuPu
20