
上浒充通大率 SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases 何谓相:物理、化学性质相同的均匀部分 相与相之间有明确界面,越过界面,性质突变 气相,无论多少,1相 •液相,纯、溶液(均匀),1相 多种液体混合,溶解度,1,2,3,… ·固相,纯或原子/分子状态相互混合成固溶体,1相 一般体系中,多一种固体便多一个相 Gas:1 phase Liquid:pure liquid or solution=1 phase Mixture of different liquids:more than 1 phases Solid:pure or atomically/molecularly distributed system,1 phase in general,one more solid=1 more phase 6
8.1 Phases Gas: 1 phase Liquid: pure liquid or solution= 1 phase Mixture of different liquids: more than 1 phases Solid: pure or atomically/molecularly distributed system, 1 phase in general, one more solid= 1 more phase 6

上浒充通大粤 SHANGHAI JIAO TONG UNIVERSITY 8.1 Phases(2) 冰、水 冰、盐水溶液 冰、盐水溶液、盐粒 冰、盐水溶液、平衡气相 Ice.water Ice,salt water Ice,salt water,salt particles Ice salt water,equilibrium vapor 7
8.1 Phases (2) Ice, water Ice, salt water Ice, salt water, salt particles Ice ,salt water, equilibrium vapor 7
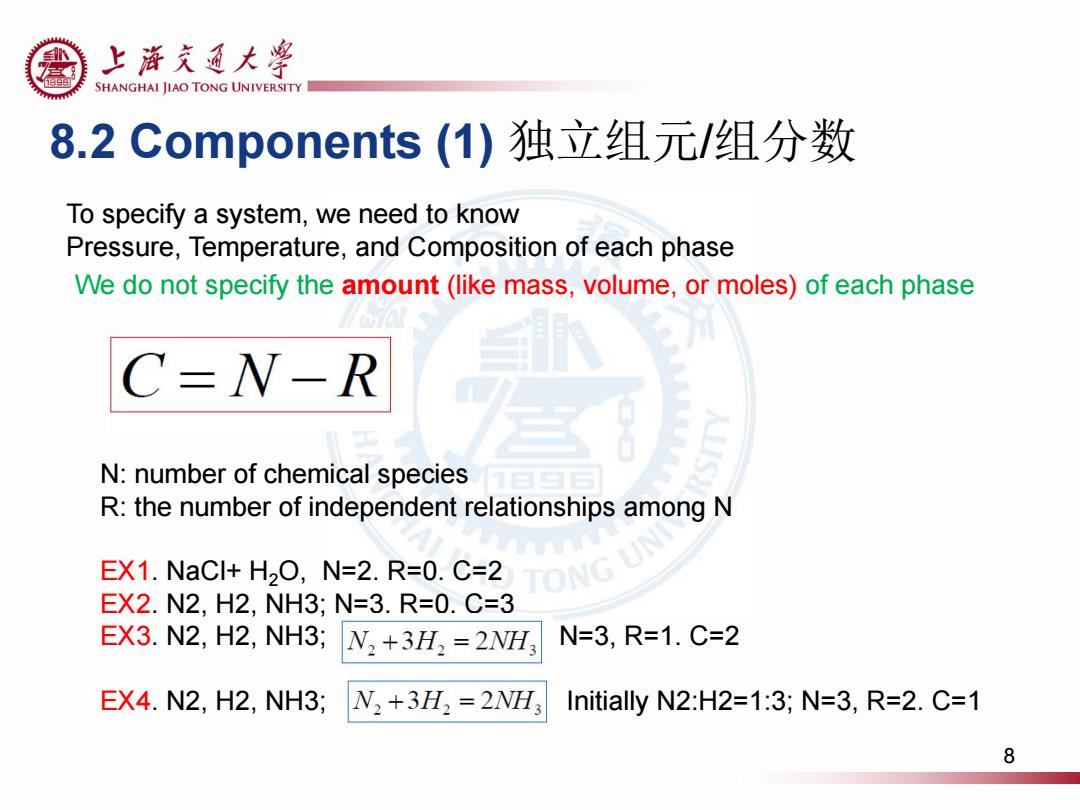
上浒充通大率 SHANGHAI JIAO TONG UNIVERSITY 8.2 Components(1)独立组元/组分数 To specify a system,we need to know Pressure,Temperature,and Composition of each phase We do not specify the amount(like mass,volume,or moles)of each phase C-N-R N:number of chemical species R:the number of independent relationships among N EX1.NaCl+H2O,N=2.R=0.C=2 TONG EX2.N2,H2,NH3;N=3.R=0.C=3 EX3.N2,H2,NH3; N,+3H,=2NH N=3,R=1.C=2 EX4.N2,H2,NH3; N2+3H2=2WH3 Initially N2:H2=1:3;N=3,R=2.C=1 8
8.2 Components (1) 独立组元/组分数 To specify a system, we need to know Pressure, Temperature, and Composition of each phase N: number of chemical species R: the number of independent relationships among N EX1. NaCl+ H2O, N=2. R=0. C=2 EX2. N2, H2, NH3; N=3, R=0. C=3 EX3. N2, H2, NH3; N=3, R=1. C=2 EX4. N2, H2, NH3; , Initially N2:H2=1:3; N=3, R=2. C=1 We do not specify the amount (like mass, volume, or moles) of each phase 8