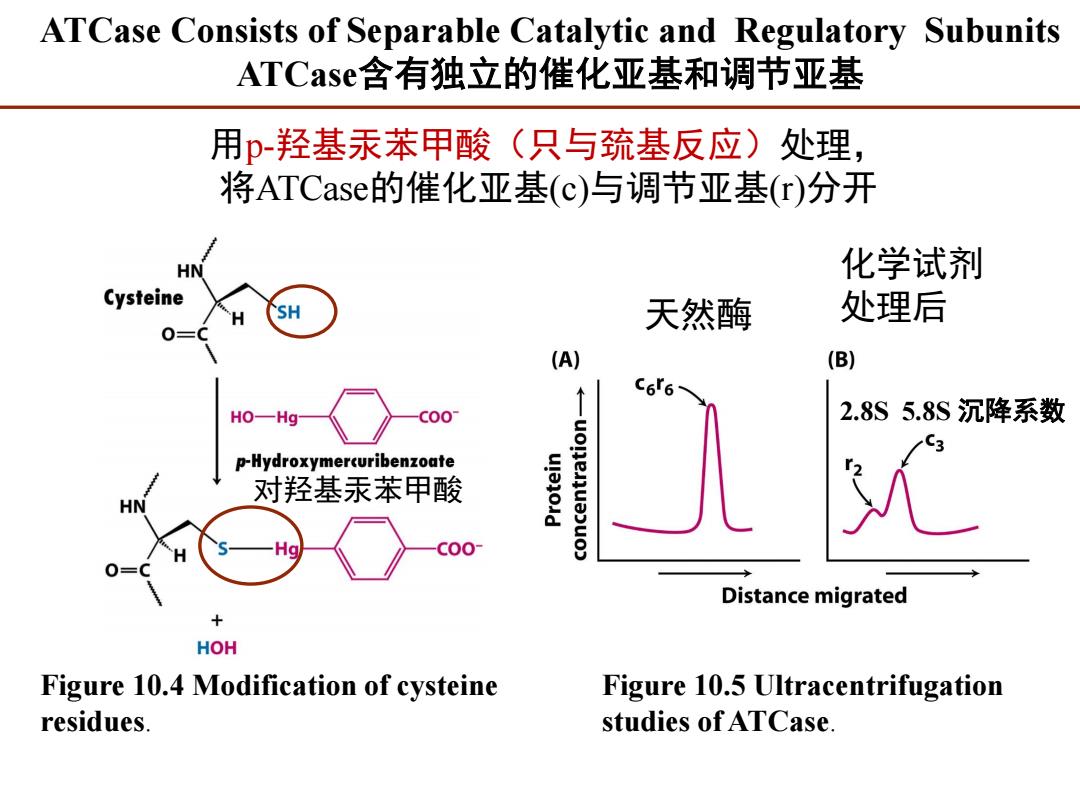
ATCase Consists of Separable Catalytic and Regulatory Subunits ATCase含有独立的催化亚基和调节亚基 用p-羟基汞苯甲酸(只与巯基反应)处理, 将ATCase的催化亚基(c)与调节亚基(r)分开 化学试剂 Cystein SH 天然酶 处理后 (A) (B) C6r6 HO一Hg C00 2.8S5.8S沉降系数 p-Hydroxymercuribenzoate 对羟基汞苯甲酸 Distance migrated + HOH Figure 10.4 Modification of cysteine Figure 10.5 Ultracentrifugation residues studies of ATCase
ATCase Consists of Separable Catalytic and Regulatory Subunits ATCase含有独立的催化亚基和调节亚基 Figure 10.4 Modification of cysteine residues. Figure 10.5 Ultracentrifugation studies of ATCase. 对羟基汞苯甲酸 用p-羟基汞苯甲酸(只与巯基反应)处理, 将ATCase的催化亚基(c)与调节亚基(r)分开 天然酶 化学试剂 处理后 2.8S 5.8S 沉降系数
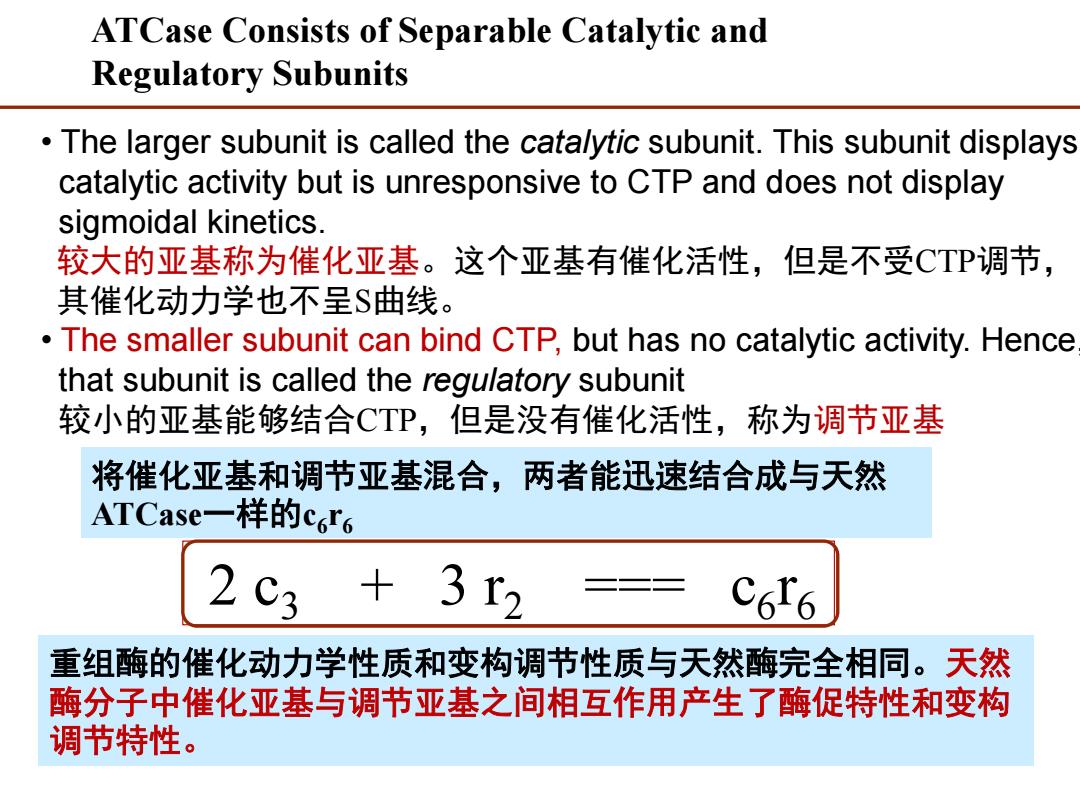
ATCase Consists of Separable Catalytic and Regulatory Subunits The larger subunit is called the cata/ytic subunit.This subunit displays catalytic activity but is unresponsive to CTP and does not display sigmoidal kinetics. 较大的亚基称为催化亚基。这个亚基有催化活性,但是不受CTP调节, 其催化动力学也不呈$曲线。 The smaller subunit can bind CTP,but has no catalytic activity.Hence that subunit is called the regulatory subunit 较小的亚基能够结合CTP,但是没有催化活性,称为调节亚基 将催化亚基和调节亚基混合,两者能迅速结合成与天然 ATCase-一样的c6r6 2 +3r2 重组酶的催化动力学性质和变构调节性质与天然酶完全相同。天然 酶分子中催化亚基与调节亚基之间相互作用产生了酶促特性和变构 调节特性
• The larger subunit is called the catalytic subunit. This subunit displays catalytic activity but is unresponsive to CTP and does not display sigmoidal kinetics. 较大的亚基称为催化亚基。这个亚基有催化活性,但是不受CTP调节, 其催化动力学也不呈S曲线。 • The smaller subunit can bind CTP, but has no catalytic activity. Hence, that subunit is called the regulatory subunit 较小的亚基能够结合CTP,但是没有催化活性,称为调节亚基 ATCase Consists of Separable Catalytic and Regulatory Subunits 2 c3 + 3 r2 === c6 r6 重组酶的催化动力学性质和变构调节性质与天然酶完全相同。天然 酶分子中催化亚基与调节亚基之间相互作用产生了酶促特性和变构 调节特性。 将催化亚基和调节亚基混合,两者能迅速结合成与天然 ATCase一样的c6 r6
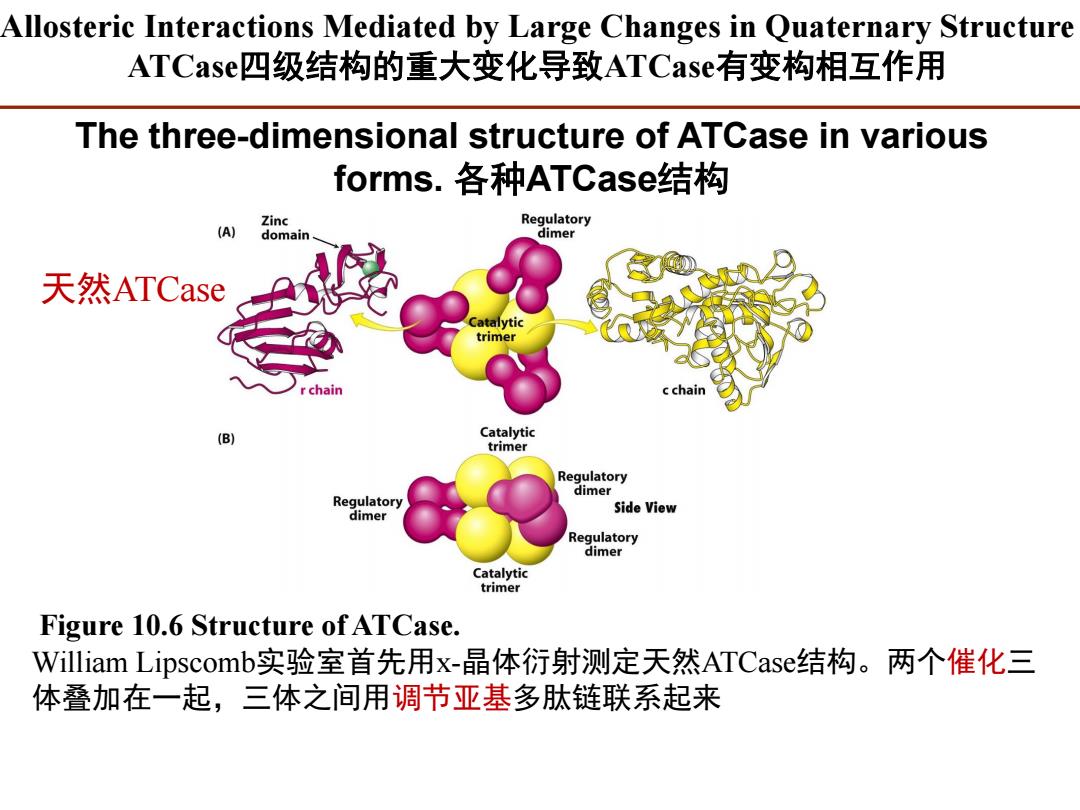
Allosteric Interactions Mediated by Large Changes in Quaternary Structure ATCase四级结构的重大变化导致ATCase有变构相互作用 The three-dimensional structure of ATCase in various forms.各种ATCase:结构 Zinc Regulatory (A) domain dimer 天然ATCase Catalytic trimer r chain c chain (B) Catalytic trimer Regulatory dimer Regulatory Side View dimer Regulatory dimer Catalytic trimer Figure 10.6 Structure of ATCase. William Lipscomb实验室首先用x-晶体衍射测定天然ATCase结构。两个催化三 体叠加在一起,三体之间用调节亚基多肽链联系起来
Allosteric Interactions Mediated by Large Changes in Quaternary Structure ATCase四级结构的重大变化导致ATCase有变构相互作用 The three-dimensional structure of ATCase in various forms. 各种ATCase结构 Figure 10.6 Structure of ATCase. William Lipscomb实验室首先用x-晶体衍射测定天然ATCase结构。两个催化三 体叠加在一起,三体之间用调节亚基多肽链联系起来 天然ATCase
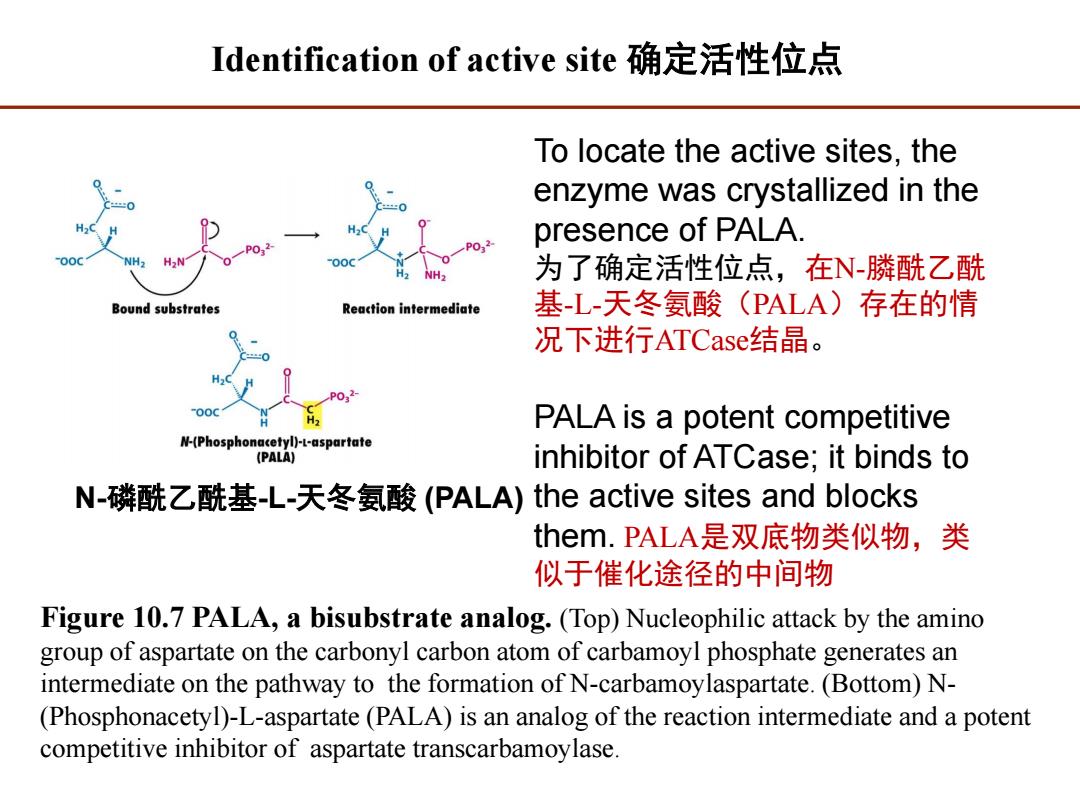
Identification of active site确定活性位点 To locate the active sites,the enzyme was crystallized in the presence of PALA. 为了确定活性位点,在N-膦酰乙酰 Bound substrates Reaction intermediate 基-L-天冬氨酸(PALA)存在的情 况下进行ATCase结晶。 PALA is a potent competitive N-(Phosphonacetyl)-L-aspartate (PALA) inhibitor of ATCase;it binds to N-磷酰乙酰基-L-天冬氨酸(PALA)the active sites and blocks them.PALA是双底物类似物,类 似于催化途径的中间物 Figure 10.7 PALA,a bisubstrate analog.(Top)Nucleophilic attack by the amino group of aspartate on the carbonyl carbon atom of carbamoyl phosphate generates an intermediate on the pathway to the formation of N-carbamoylaspartate.(Bottom)N- (Phosphonacetyl)-L-aspartate(PALA)is an analog of the reaction intermediate and a potent competitive inhibitor of aspartate transcarbamoylase
Figure 10.7 PALA, a bisubstrate analog. (Top) Nucleophilic attack by the amino group of aspartate on the carbonyl carbon atom of carbamoyl phosphate generates an intermediate on the pathway to the formation of N-carbamoylaspartate. (Bottom) N- (Phosphonacetyl)-L-aspartate (PALA) is an analog of the reaction intermediate and a potent competitive inhibitor of aspartate transcarbamoylase. To locate the active sites, the enzyme was crystallized in the presence of PALA. 为了确定活性位点,在N-膦酰乙酰 基-L-天冬氨酸(PALA)存在的情 况下进行ATCase结晶。 PALA is a potent competitive inhibitor of ATCase; it binds to the active sites and blocks them. PALA是双底物类似物,类 似于催化途径的中间物 N-磷酰乙酰基-L-天冬氨酸 (PALA) Identification of active site 确定活性位点
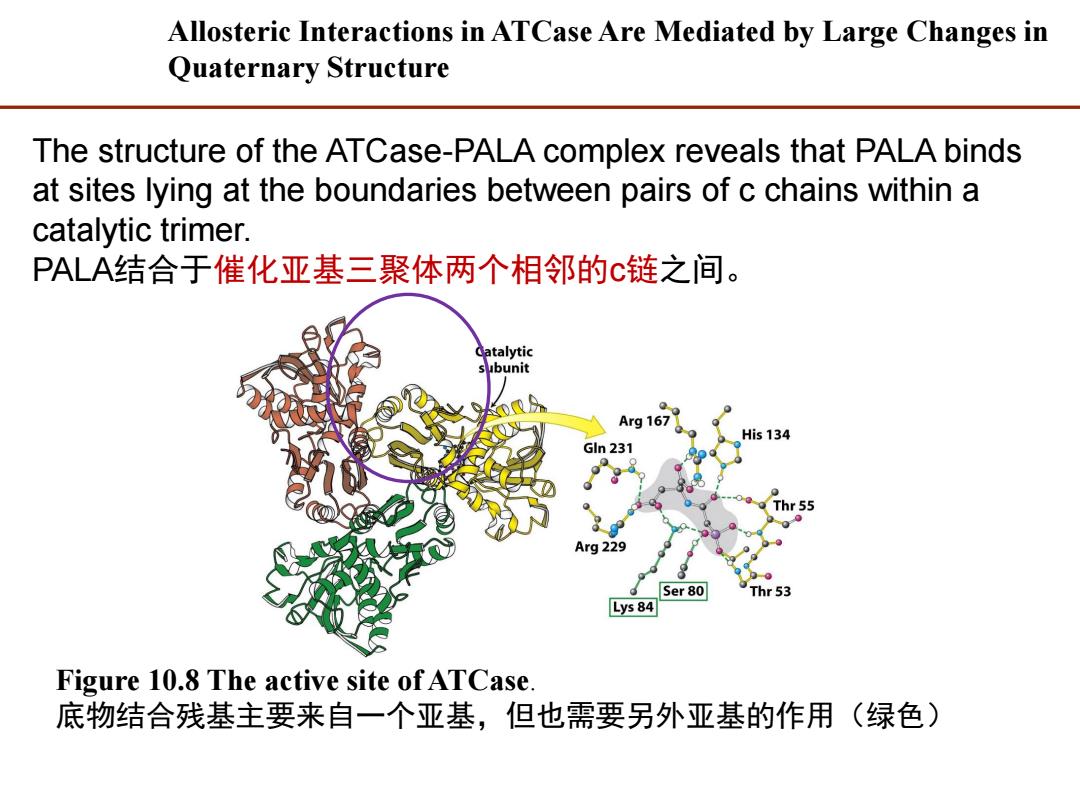
Allosteric Interactions in ATCase Are Mediated by Large Changes in Quaternary Structure The structure of the ATCase-PALA complex reveals that PALA binds at sites lying at the boundaries between pairs of c chains within a catalytic trimer. PALA结合于催化亚基三聚体两个相邻的c链之间。 Catalytic subunit Arg 167 His 134 Gln 231 Thr 55 Arg229 Ser80 Thr53 Lys 84 Figure 10.8 The active site of ATCase. 底物结合残基主要来自一个亚基,但也需要另外亚基的作用(绿色)
Figure 10.8 The active site of ATCase. 底物结合残基主要来自一个亚基,但也需要另外亚基的作用(绿色) The structure of the ATCase-PALA complex reveals that PALA binds at sites lying at the boundaries between pairs of c chains within a catalytic trimer. PALA结合于催化亚基三聚体两个相邻的c链之间。 Allosteric Interactions in ATCase Are Mediated by Large Changes in Quaternary Structure