
幻灯片26 z7 当电路中无电流通过时的电池具有最大电压(开路电压),当电路中电流趋于零时,两电极间的最大电势差-一一-一电池电动势吓 0letrotive force)
幻灯片 26 z7 当电路中无电流通过时的电池具有最大电压(开路电压),当电路中电流趋于零时,两电极间的最大电势差------电池电动势EMF (Emf:electromotive force) zhang, 2007-10-28
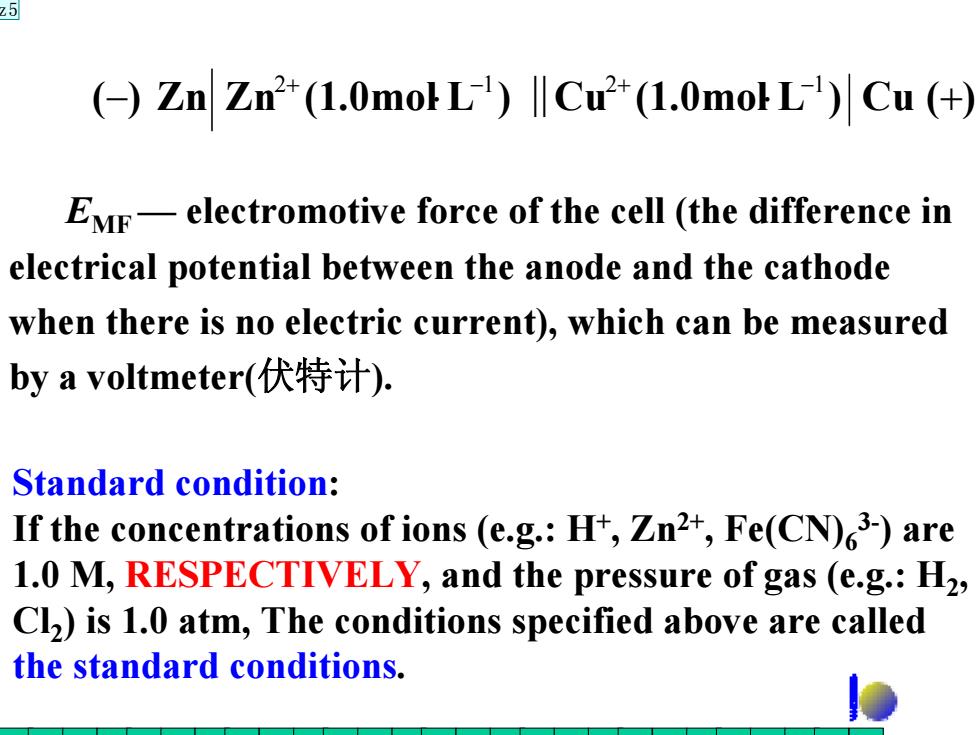
25 (Zn Zn2 (1.0mokL)Cu(1.0moL)Cu (+ EMF-electromotive force of the cell (the difference in electrical potential between the anode and the cathode when there is no electric current),which can be measured by a voltmeter(伏特计). Standard condition: If the concentrations of ions (e.g.:H+,Zn2+,Fe(CN)3-)are 1.0 M,RESPECTIVELY,and the pressure of gas (e.g.:H2, Cl)is 1.0 atm,The conditions specified above are called the standard conditions
EMF — electromotive force of the cell (the difference in electrical potential between the anode and the cathode when there is no electric current), which can be measured by a voltmeter(伏特计). (−) Zn Zn (1.0mol⋅L ) Cu (1.0mol⋅L ) Cu (+) 2+ −1 ‖ 2+ −1 Standard condition: If the concentrations of ions (e.g.: H+, Zn2+, Fe(CN)63-) are 1.0 M, RESPECTIVELY, and the pressure of gas (e.g.: H2, Cl2) is 1.0 atm, The conditions specified above are called the standard conditions. z5

幻灯片27 当电路中无电流通过时的电池具有最大电压(开路电压),当电路中电流趋于零时,两电极间的最大电势差-一一一一电池电动势 (Emf:electromotive force) zb0,2010-03-03
幻灯片 27 z5 当电路中无电流通过时的电池具有最大电压(开路电压),当电路中电流趋于零时,两电极间的最大电势差------电池电动势EMF (Emf:electromotive force) zho, 2010-03-03

Evr-standard electromotive force. the voltage difference EMr between the two electrodes which are under standard conditions. For example,the Cu/Zn cell at standard conditions will deliver 1.10v,i.e.,Ev=1.10V
EMFΘ ⎯ standard electromotive force. the voltage difference EMF between the two electrodes which are under standard conditions. For example, the Cu/Zn cell at standard conditions will deliver 1.10V, i.e., EMFΘ = 1.10V
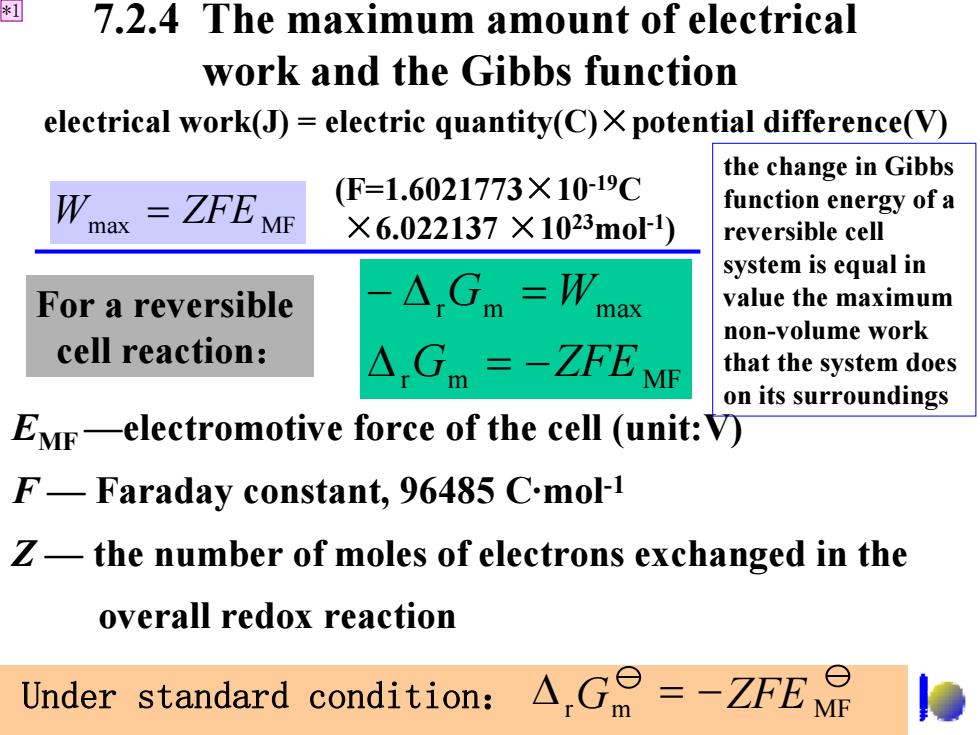
* 7.2.4 The maximum amount of electrical work and the Gibbs function electrical work(J)=electric quantity(C)X potential difference(V) the change in Gibbs W ZFE ME (F=1.6021773×10-19C function energy of a max ×6.022137×1023mol) reversible cell system is equal in For a reversible △Gm=W ax value the maximum non-volume work cell reaction: △Gm=-ZFEMF that the system does on its surroundings EMr-electromotive force of the cell (unit:V) F-Faraday constant,96485 C.mol-1 Z-the number of moles of electrons exchanged in the overall redox reaction Under standard condition: △,G=-ZFE
7.2.4 The maximum amount of electrical work and the Gibbs function EMF —electromotive force of the cell (unit:V) F — Faraday constant, 96485 C·mol-1 Z — the number of moles of electrons exchanged in the overall redox reaction r m MF r m max G ZFE G W Δ = − − Δ = Wmax = ZFE MF electrical work(J) = electric quantity(C) ×potential difference(V) For a reversible cell reaction : Under standard condition: r G m ZFE MF Δ = − (F=1.6021773 ×10-19 C ×6.022137 ×1023mol-1 ) the change in Gibbs function energy of a reversible cell system is equal in value the maximum non-volume work that the system does on its surroundings *1