
先进材料疑固实验室 Laboratory of Advanced Materials Solidification 1896 1920 1987 2006 ciquidmeta metal with the atomle structure oraliquld Nucleation in solidification(2) Dr.Mingxu Xia anced Mate 周 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
1896 1920 1987 2006 Nucleation in solidification (2) Dr. Mingxu Xia
先进材料疑固实验室 Outline Laboratory of Advanced Materials Solidification Solidification Concepts: >Definition >Nucleation free energy Thermo performance >Nucleation barrier Conventional Nucleation Theory >Critical nucleus >Homogeneous nucleation Heterogeneous nucleation >Nucleation rate Nucleation rate and its control Nucleation rate >Grain refining >Metallic glass Further Nucleation in atomic level Non-Conventional Nucleation Theory nced 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
Outline Solidification Definition Thermo performance Conventional Nucleation Theory Homogeneous nucleation Heterogeneous nucleation Nucleation rate and its control Nucleation rate Grain refining Metallic glass Further Nucleation in atomic level Non-Conventional Nucleation Theory Concepts: Nucleation free energy Nucleation barrier Critical nucleus Nucleation rate

先进材料疑固实验室 Nucleation rate and its control Laboratory of Advanced Materials Solidification Nucleation rate is the number of nucleation events per unit volume per unit time. For the nucleation of a solid crystal to occur,one of the fluctuating solid embryos needs to reach a radius being equal or greater than Rc. The rate for this event to occur is the nucleation rate,1. 上降充通大学 SHANGHAI JIAO TONG UNIVERSITY
Nucleation rate and its control • Nucleation rate is the number of nucleation events per unit volume per unit time. • For the nucleation of a solid crystal to occur, one of the fluctuating solid embryos needs to reach a radius being equal or greater than Rc. • The rate for this event to occur is the nucleation rate, I
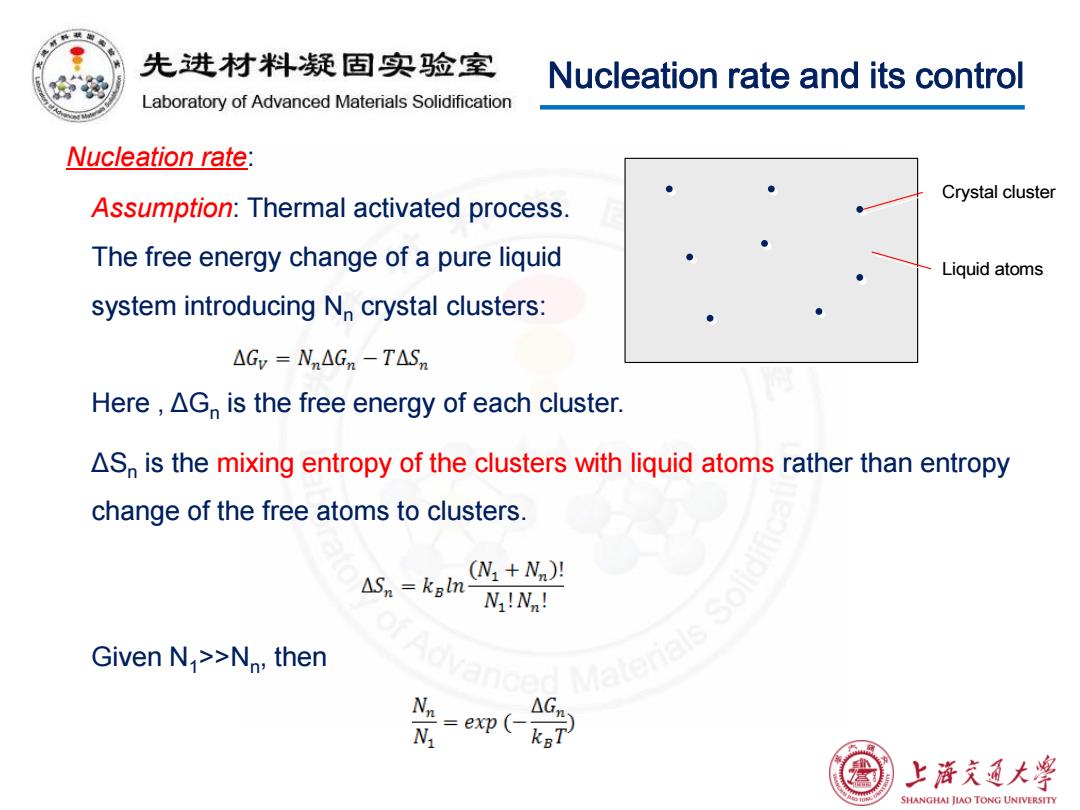
先进材料疑固实验室 Nucleation rate and its control Laboratory of Advanced Materials Solidification Nucleation rate: Crystal cluster Assumption:Thermal activated process. The free energy change of a pure liquid Liquid atoms system introducing N crystal clusters: △Gv-Nn△Gn-T△Sm Here,AG is the free energy of each cluster. AS is the mixing entropy of the clusters with liquid atoms rather than entropy change of the free atoms to clusters. △Sn=kal (N1+Nn)! N!N! Given N >>Nn,then G =ep( 上降充通大学 SHANGHAI JIAO TONG UNIVERSITY
Nucleation rate and its control Nucleation rate: Assumption: Thermal activated process. The free energy change of a pure liquid system introducing Nn crystal clusters: Here , ΔGn is the free energy of each cluster. Crystal cluster Liquid atoms ΔSn is the mixing entropy of the clusters with liquid atoms rather than entropy change of the free atoms to clusters. Given N1>>Nn , then
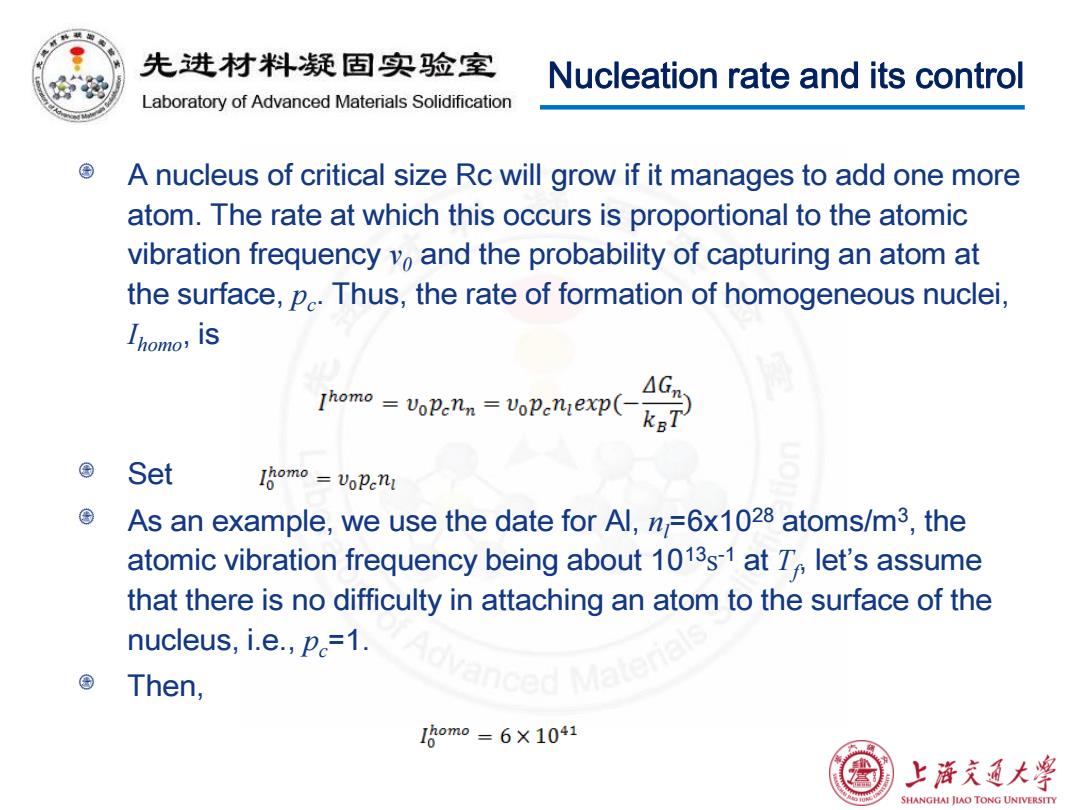
积 先进材料疑固实验室 Nucleation rate and its control Laboratory of Advanced Materials Solidification A nucleus of critical size Rc will grow if it manages to add one more atom.The rate at which this occurs is proportional to the atomic vibration frequency vo and the probability of capturing an atom at the surface,p.Thus,the rate of formation of homogeneous nuclei, Ihomo,iS Thomo =VoPenn VoPeniexp( △G Set Iomo=voPenI As an example,we use the date for Al,n=6x1028 atoms/m3,the atomic vibration frequency being about 1013s-1 at T let's assume that there is no difficulty in attaching an atom to the surface of the nucleus,i.e.,p=1. Then, I8om0=6×1041 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
Nucleation rate and its control A nucleus of critical size Rc will grow if it manages to add one more atom. The rate at which this occurs is proportional to the atomic vibration frequency ν0 and the probability of capturing an atom at the surface, pc . Thus, the rate of formation of homogeneous nuclei, Ihomo, is Set As an example, we use the date for Al, nl=6x1028 atoms/m3 , the atomic vibration frequency being about 1013s -1 at Tf , let’s assume that there is no difficulty in attaching an atom to the surface of the nucleus, i.e., pc=1. Then