
先进材料疑固实验室 Laboratory of Advanced Materials Solidification 1896 1920 1987 2006 Crystal growth of single element solids(3) Dr.Mingxu Xia anced Mat 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
1896 1920 1987 2006 Crystal growth of single element solids (3) Dr. Mingxu Xia

先进材料疑固实验室 OUTLINE Laboratory of Advanced Materials Solidification Liquid/solid interface Crystal growth of single element solids Types of crystal growth ·Kinetics of growth Morphology control of crystals Crystal growth methods(technology) ory of vanced Materials Solid 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
OUTLINE Liquid/solid interface Crystal growth of single element solids • Types of crystal growth • Kinetics of growth • Morphology control of crystals Crystal growth methods (technology)
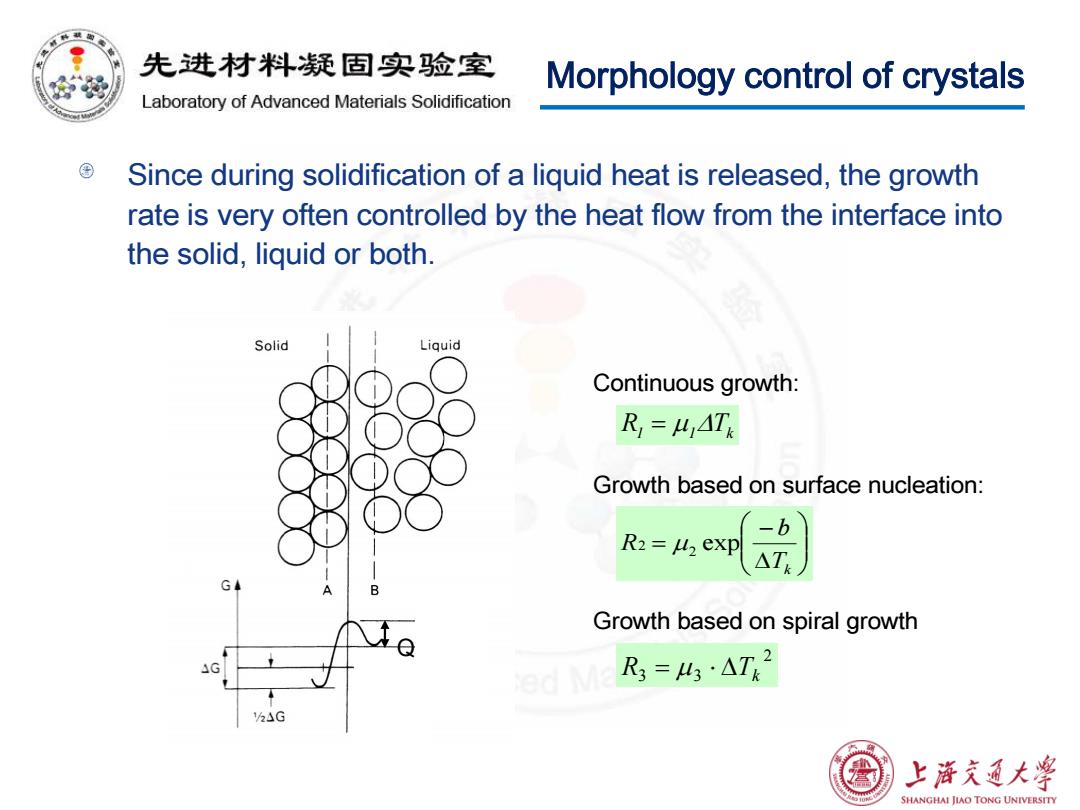
先进材料疑固实验室 Morphology control of crystals Laboratory of Advanced Materials Solidification Since during solidification of a liquid heat is released,the growth rate is very often controlled by the heat flow from the interface into the solid,liquid or both. Solid Liquid Continuous growth: R=LAT Growth based on surface nucleation: -b R2 M2 exp △T Growth based on spiral growth 4G ed Ma 3=43△T2 h△G 熟 上海充通大¥ SHANGHAI JIAO TONG UNIVERSITY
Morphology control of crystals Since during solidification of a liquid heat is released, the growth rate is very often controlled by the heat flow from the interface into the solid, liquid or both. Q ∆ − = Tk b R2 µ2 exp 2 R3 3 ∆Tk = µ ⋅ R1 = µ1∆Tk Continuous growth: Growth based on surface nucleation: Growth based on spiral growth
先进材料疑固实验室 Morphology control of crystals Laboratory of Advanced Materials Solidification What is the mechanisms of the effect of heat flow on crystal growth? After nucleation,normally AT;is fairly large,and this causes high growth rate and rapid heat release. If the (negative)temperature gradient in the solid or the liquid is not sufficiently large,the heat cannot be transferred away quickly enough, and the residual heat is used to heat the solid and liquid near the S/L interface. This will cause an increase of Ti,and a decrease of ATi,which in turn leads to a decrease of growth rate and the rate of heat release from solidification. At some point,these two opposite effects are balanced,and ATi remains unchanged.This is called steady state for crystal growth. T;and AT;are the temperature and undercooling at the interface 上海充通大¥ SHANGHAI JIAO TONG UNIVERSITY
Morphology control of crystals What is the mechanisms of the effect of heat flow on crystal growth? • After nucleation, normally ∆Ti is fairly large, and this causes high growth rate and rapid heat release. • If the (negative) temperature gradient in the solid or the liquid is not sufficiently large, the heat cannot be transferred away quickly enough, and the residual heat is used to heat the solid and liquid near the S/L interface. • This will cause an increase of Ti , and a decrease of ∆Ti , which in turn leads to a decrease of growth rate and the rate of heat release from solidification. • At some point, these two opposite effects are balanced, and ∆Ti remains unchanged. This is called steady state for crystal growth. Ti and ∆Ti are the temperature and undercooling at the interface

先进材料疑固实验室 Morphology control of crystals Laboratory of Advanced Materials Solidification Normally,in steady state,AT;is fairly small,and less than 1K. The temperature gradient in the liquid in front of the S/L interface has a significant effect on the stability of S/L interface. Positive temperature gradient in the liquid>stable S/L interface. Negative temperature gradient in the liquid>unstable S/L interface and formation of solid dendrites. For single element solids,the morphology of the crystal depends on microstructure of the interface(surface type)and the temperature distribution in front of the interface. 上浒充通大学 SHANGHAI JIAO TONG UNIVERSITY
Morphology control of crystals Normally, in steady state, ∆Ti is fairly small, and less than 1K. The temperature gradient in the liquid in front of the S/L interface has a significant effect on the stability of S/L interface. • Positive temperature gradient in the liquid stable S/L interface. • Negative temperature gradient in the liquid unstable S/L interface and formation of solid dendrites. For single element solids, the morphology of the crystal depends on microstructure of the interface (surface type) and the temperature distribution in front of the interface