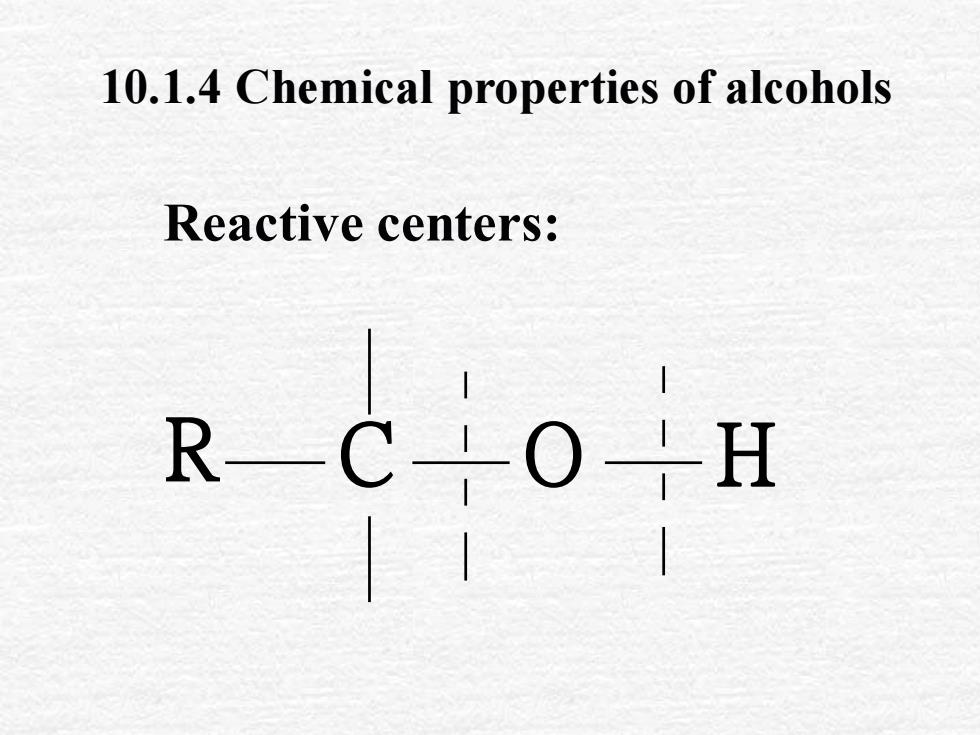
10.1.4 Chemical properties of alcoholsReactive centers:R-C-H0-
10.1.4 Chemical properties of alcohols Reactive centers: R C O H
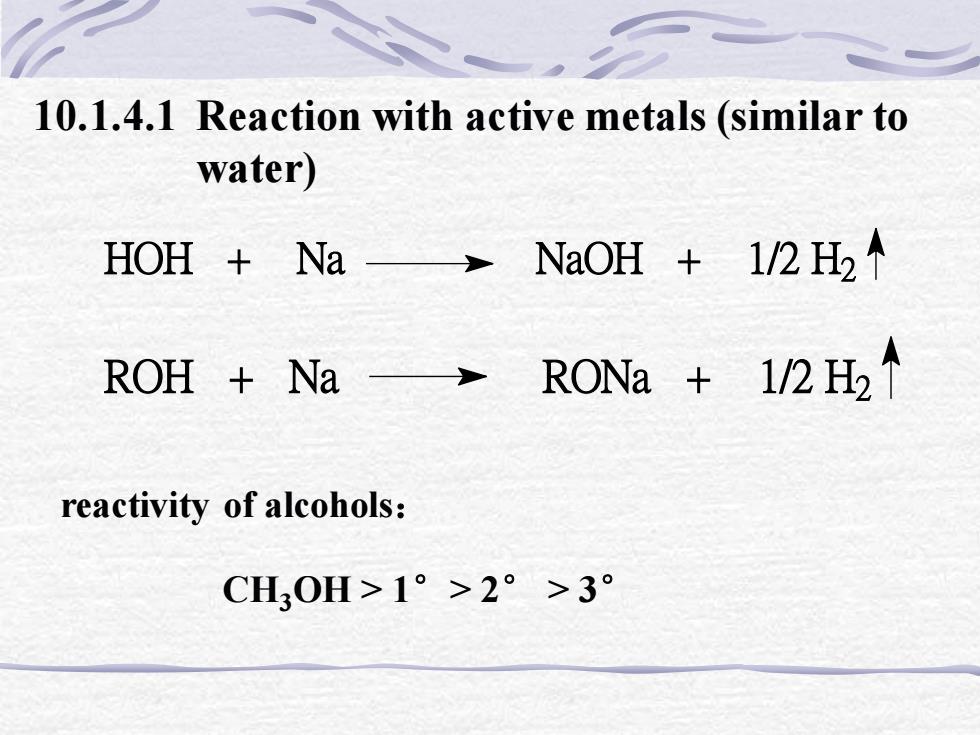
10.1.4.1 Reaction with active metals (similar towater)HOH + Na - → NaOH + 1/2 H2 RONa +1/2 H2ROH + Nareactivity of alcohols:CH,0H>1° >2° >3°
10.1.4.1 Reaction with active metals (similar to water) reactivity of alcohols: CH3OH > 1°> 2° > 3° HOH + Na NaOH + 1/2 H2 ROH + Na RONa + 1/2 H2
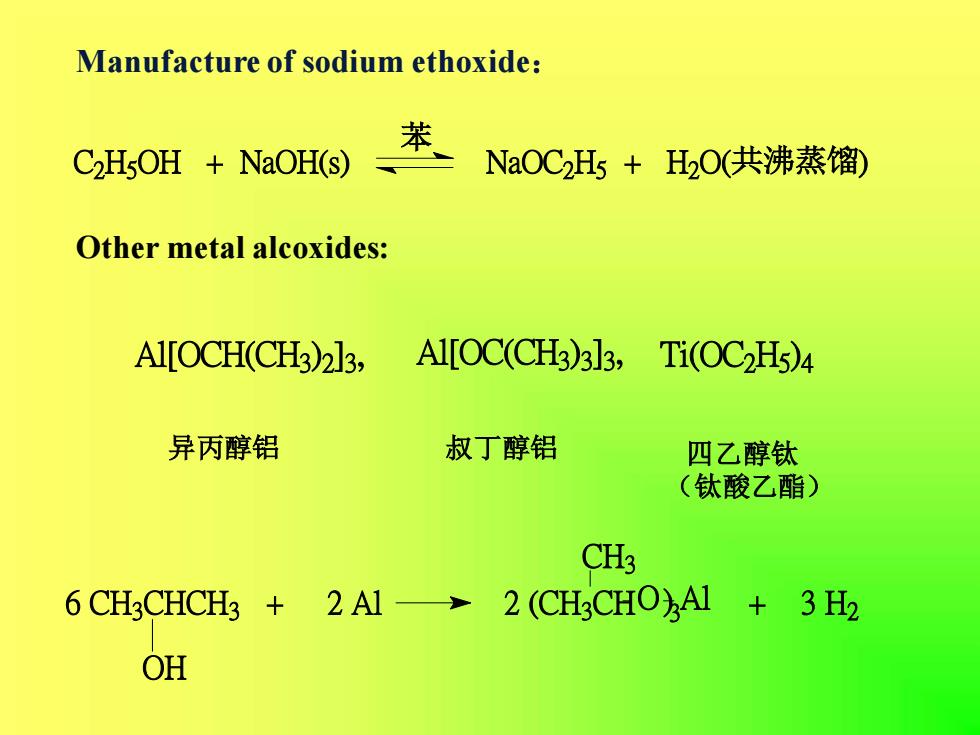
Manufacture of sodium ethoxide:苯NaOC2Hs + H2O(共沸蒸馏)C2H5OH + NaOH(s)OthermetalalcoxidessAl[OC(CH3)3]3,Al[OCH(CH3)2]3,Ti(OC2Hs)4异丙醇铝叔丁醇铝四乙醇钛(钛酸乙酯)CH36 CH3CHCH3 + 2 Al → 2(CH3CHO)Al + 3 H2OH
Manufacture of sodium ethoxide: Other metal alcoxides: 异丙醇铝 四乙醇钛 (钛酸乙酯) 叔丁醇铝 C2 H5 OH + NaOH(s) NaOC2 H5 + H2 O(共沸蒸馏) 苯 Al[OCH(CH3 ) 2 ] 3 , Al[OC(CH3 ) 3 ] 3 , Ti(OC2 H5 ) 4 6 CH3 CHCH3 + 2 Al 2 (CH3 CH + 3 H2 OH O) Al 3 CH3
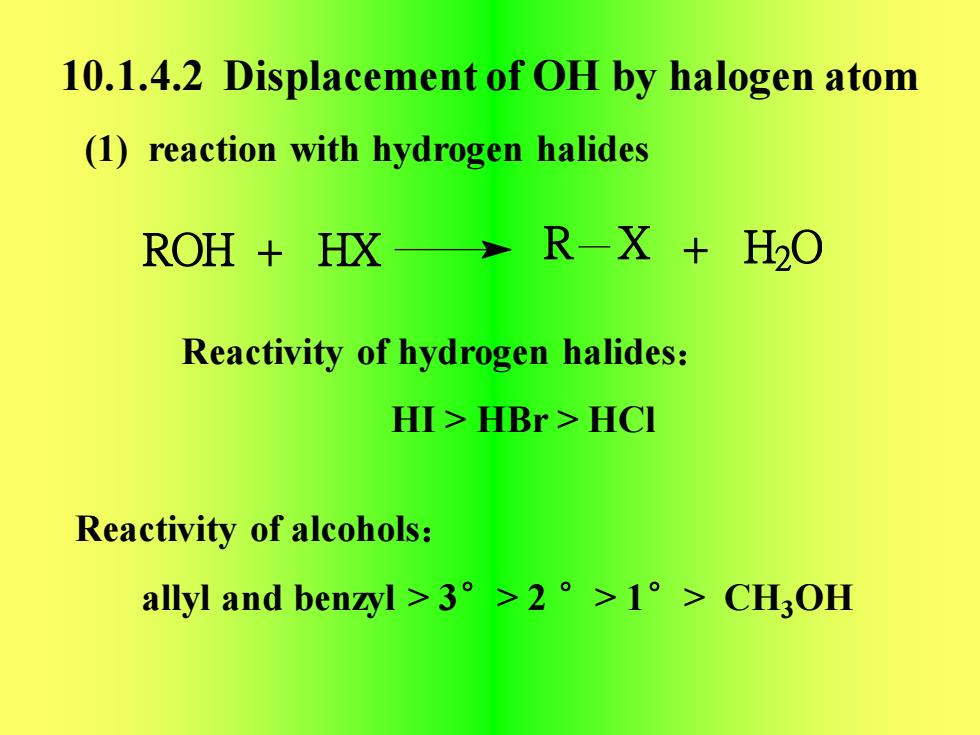
10.1.4.2Displacementof OH byhalogenatom(1) reaction with hydrogen halides R-X + H20ROH + HXReactivity of hydrogen halides:HI>HBr>HCIReactivity of alcohols:allyl and benzyl >3°>2 °>1°> CHOH
10.1.4.2 Displacement of OH by halogen atom (1) reaction with hydrogen halides Reactivity of hydrogen halides: HI > HBr > HCl Reactivity of alcohols: allyl and benzyl > 3°> 2 °> 1°> CH3OH ROH + HX R X + H2 O
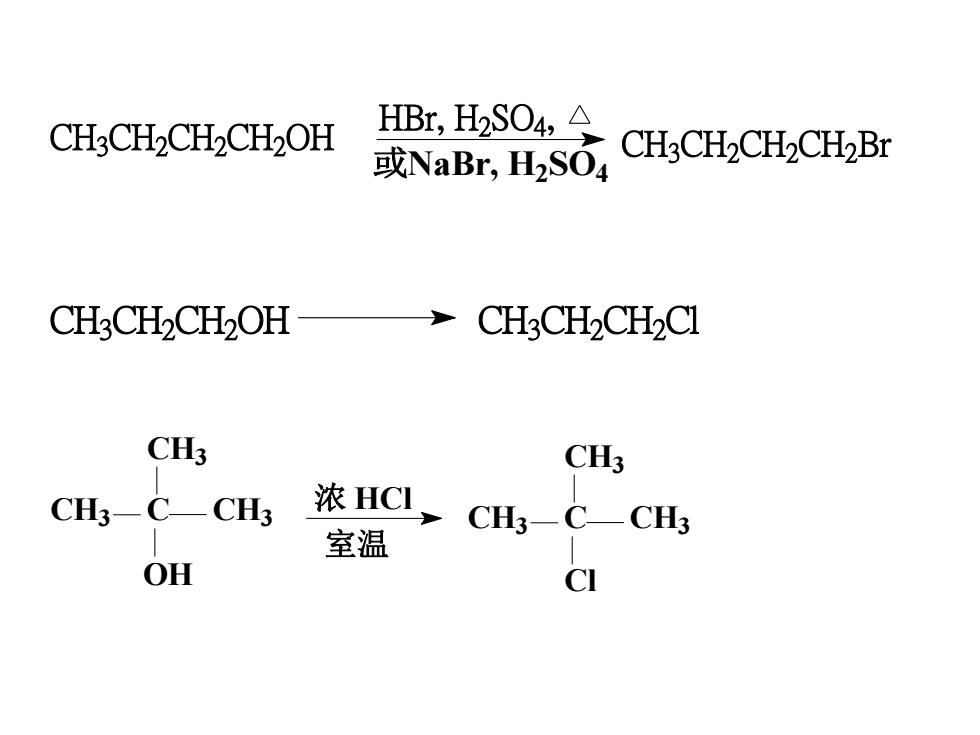
HBr, H2SO4, CH3CH2CH2CH2OHCH3CH2CH2CH2Br或NaBr, H2SO4CH3CH2CH2OH CH3CH2CH2ClCH3CH3浓 HCICH3—C—CH3CH3_CH3室温OHCl
CH3 CH2 CH2 CH2 OH CH3 CH2 CH2 CH2 Br HBr, H2 SO4 , 或NaBr, H2 SO4 CH3 CH2 CH2 OH CH3 CH2 CH2 Cl CH3 C CH3 OH CH3 CH3 C CH3 CH3 Cl 浓 HCl 室温