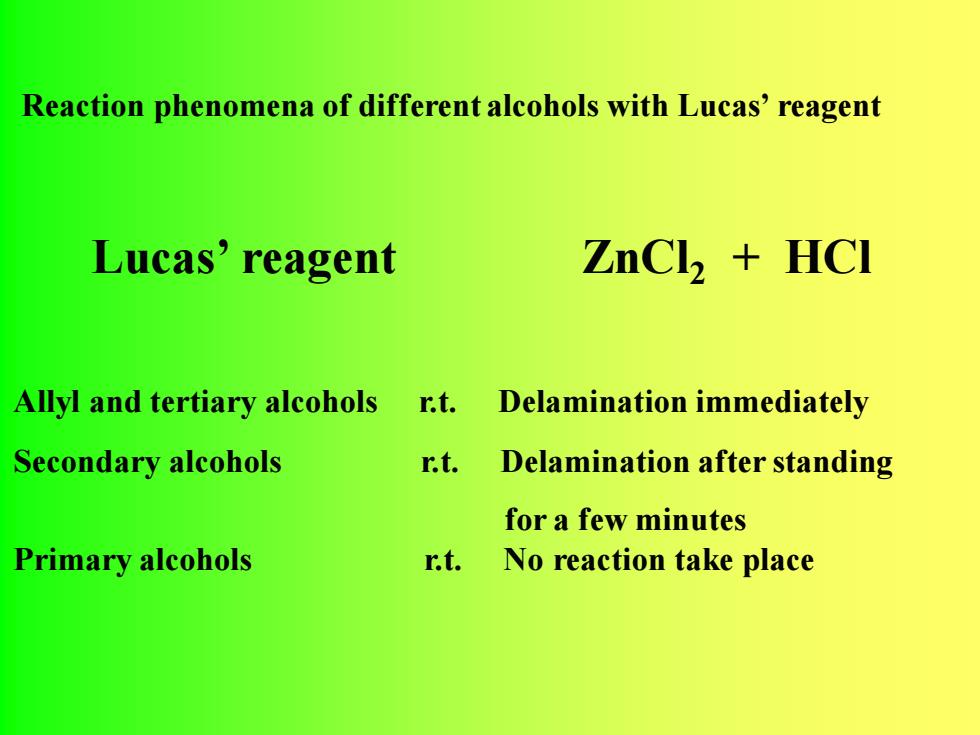
Reaction phenomena of different alcohols with Lucas'reagentZnCl + HCILucas' reagentAllyl andtertiaryalcoholsDelaminationimmediatelyr.t.Secondary alcoholsr.t.Delamination afterstandingfor a few minutesPrimaryalcoholsr.t. No reaction take place
Allyl and tertiary alcohols r.t. Delamination immediately Secondary alcohols r.t. Delamination after standing for a few minutes Primary alcohols r.t. No reaction take place Reaction phenomena of different alcohols with Lucas’ reagent Lucas’ reagent ZnCl2 + HCl
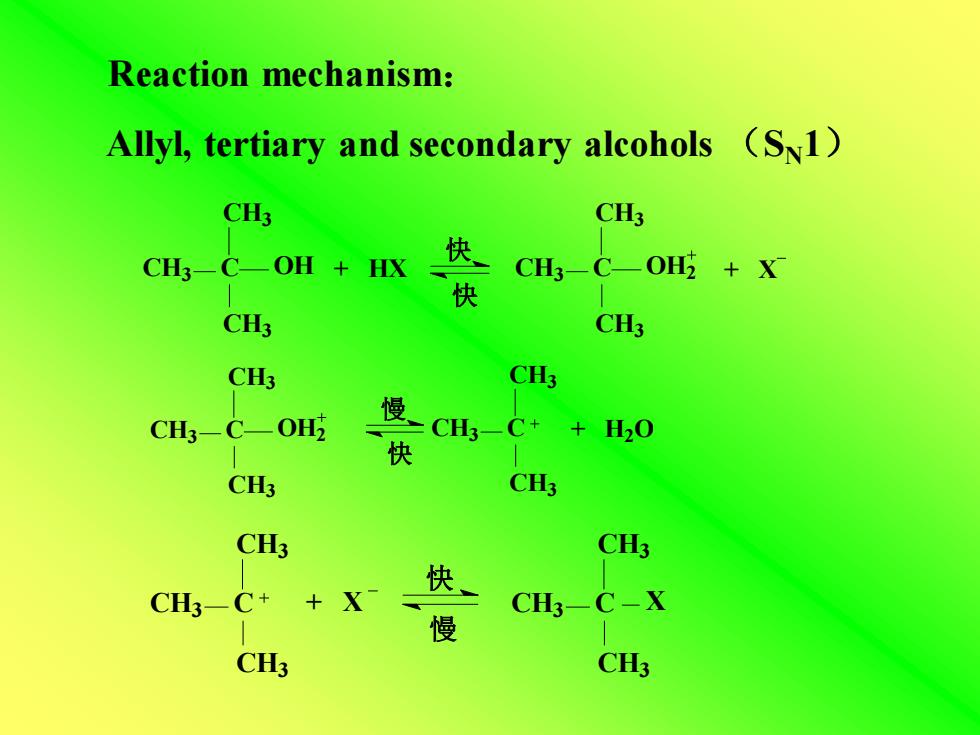
Reaction mechanism:Allyl, tertiary and secondary alcohols (Sn1)CH3CH3快OHOHCH3+HXCH-C+X快CH3CH3CH3CH3慢OH2CH3C+CH3—CXH,0快CH3CH3CH3CH3快+ XCH3-C+CH3-C-X慢CH3CH3
Allyl, tertiary and secondary alcohols (SN1) Reaction mechanism: CH3 C OH CH3 CH3 CH3 C OH2 CH3 CH3 + HX + X 快 快 CH3 C OH2 CH3 CH3 慢 快 CH3 C + H2O CH3 CH3 CH3 C CH3 CH3 慢 快 + X CH3 C CH3 CH3 X
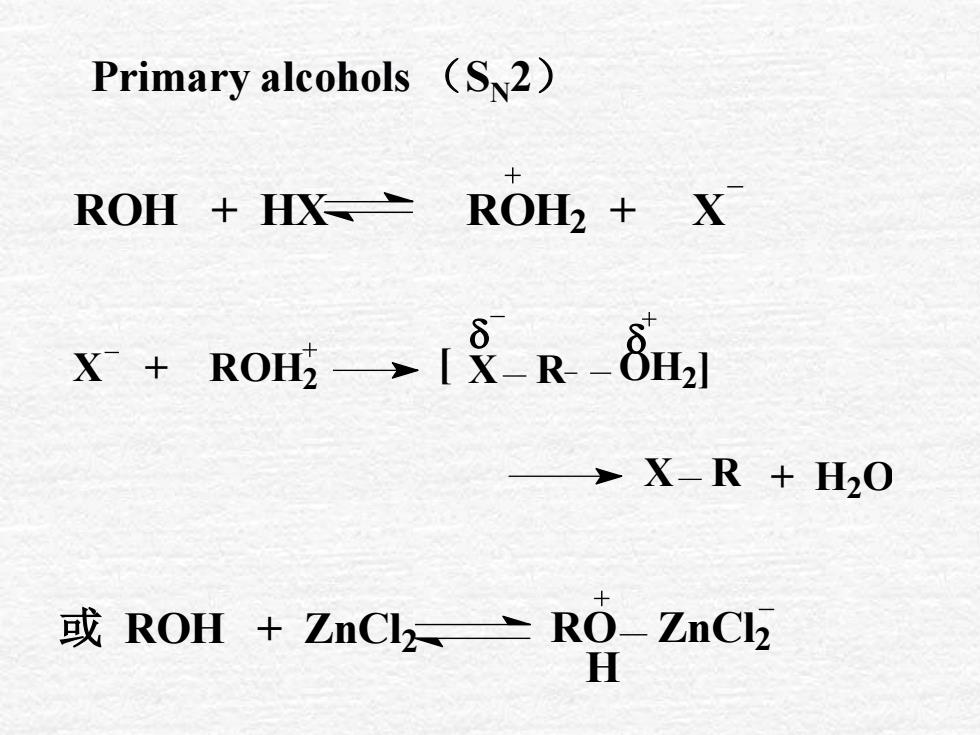
Primary alcohols (Sμ2)ROH2 + XROH + HX?X + ROH, →I&-R -8H]→ X-R + H0或 ROH + ZnClz RO- ZnClH
Primary alcohols (SN2) ROH + HX ROH2 + X X + ROH2 [ X R OH2] X R + H2 O 或 ROH + ZnCl 2 RO ZnCl 2 H
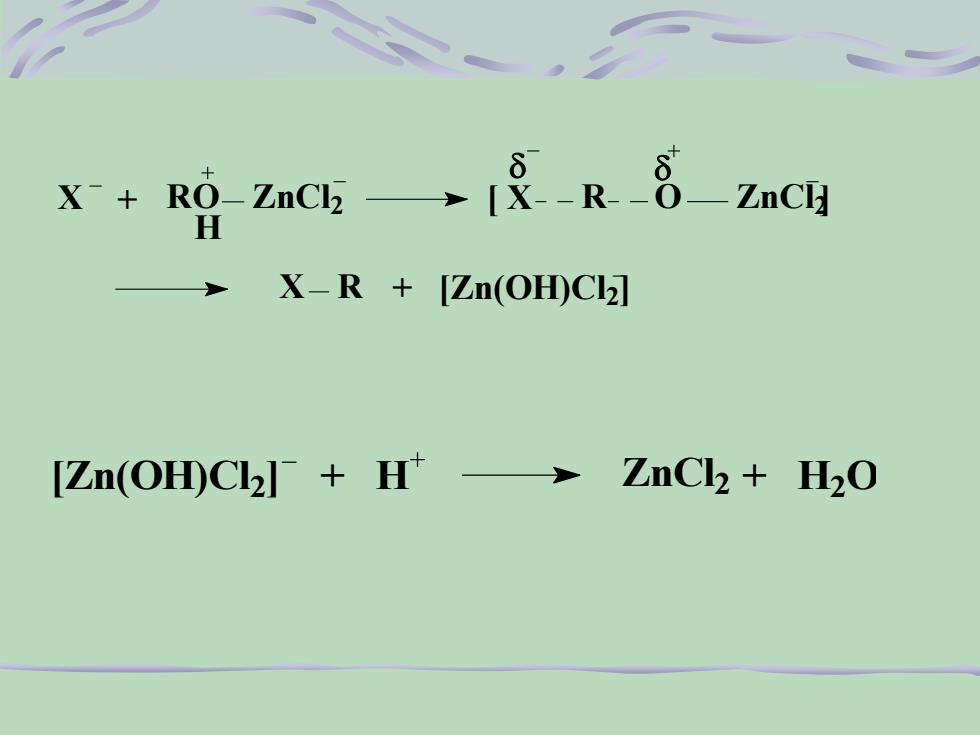
8O→[α--R--8—ZnCIX- + RO-ZnCl -HX-R + [Zn(OH)C][Zn(OH)Clh]- + H+ → ZnCl + H,O
RO ZnCl 2 H X + [ X R O ZnCl 2] X R + [Zn(OH)Cl 2] [Zn(OH)Cl 2 ] + H ZnCl 2 + H2 O
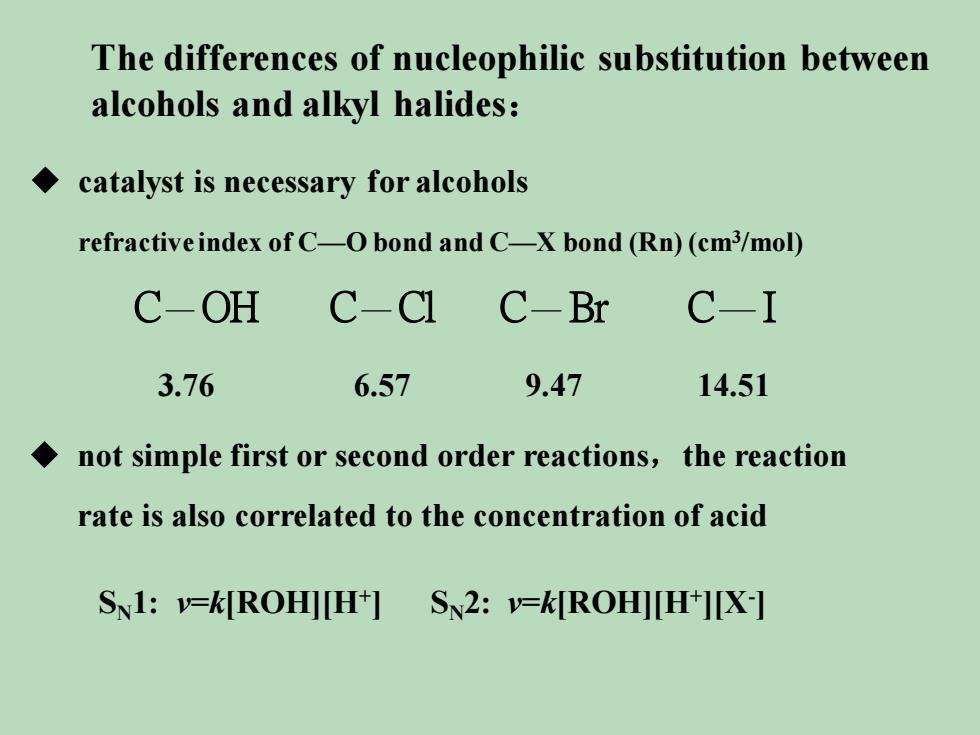
The differences of nucleophilic substitution betweenalcohols and alkyl halides:catalyst is necessary foralcoholsrefractiveindexofCObond and CXbond (Rn)(cm3/mol)C-IC-OHC-ClC-Br6.579.473.7614.51not simple first or second order reactions, the reactionrate is also correlated to the concentration of acidSN1-k[ROH|[H] S2:-k[ROH[H+I[X]
The differences of nucleophilic substitution between alcohols and alkyl halides: ◆ catalyst is necessary for alcohols refractive index of C—O bond and C—X bond (Rn) (cm3 /mol) 3.76 6.57 9.47 14.51 ◆ not simple first or second order reactions,the reaction rate is also correlated to the concentration of acid SN1: v=k[ROH][H+ ] SN2: v=k[ROH][H+ ][X- ] C OH C Cl C Br C I