
Chapter 2,part B Chemical Principles
Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings B.E Pruitt & Jane J. Stein Chapter 2, part B Chemical Principles
Important Biological Molecules Organic compounds always contain carbon and hydrogen. Inorganic compounds typically lack carbon
Important Biological Molecules • Organic compounds always contain carbon and hydrogen. • Inorganic compounds typically lack carbon
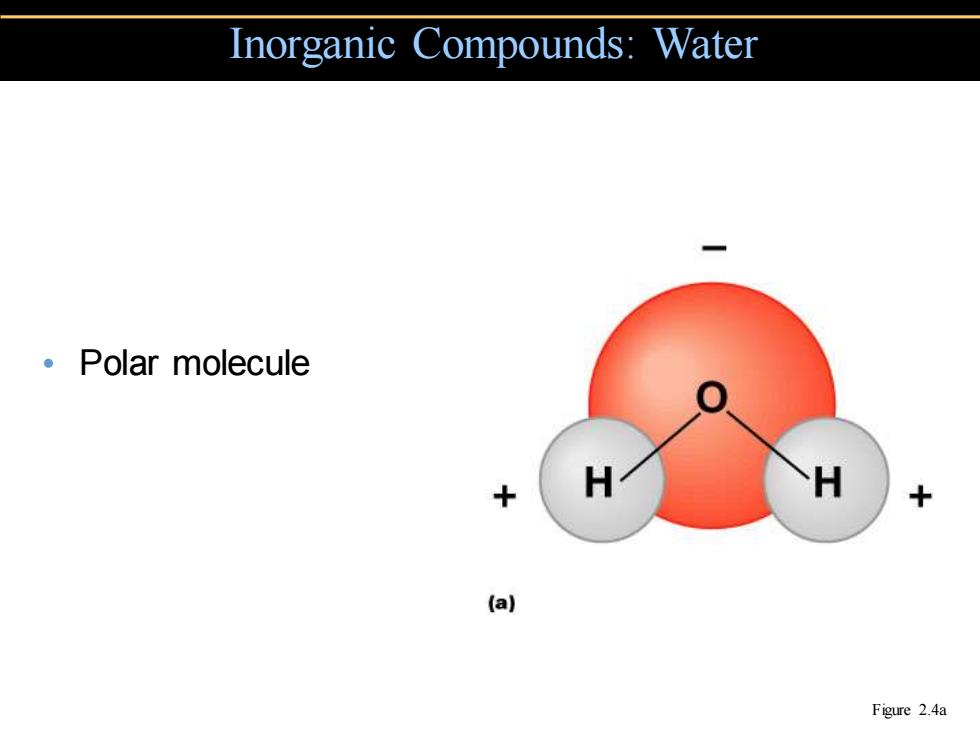
Inorganic Compounds:Water 。Polar molecule H (a) Figure 2.4a
• Polar molecule Inorganic Compounds: Water Figure 2.4a
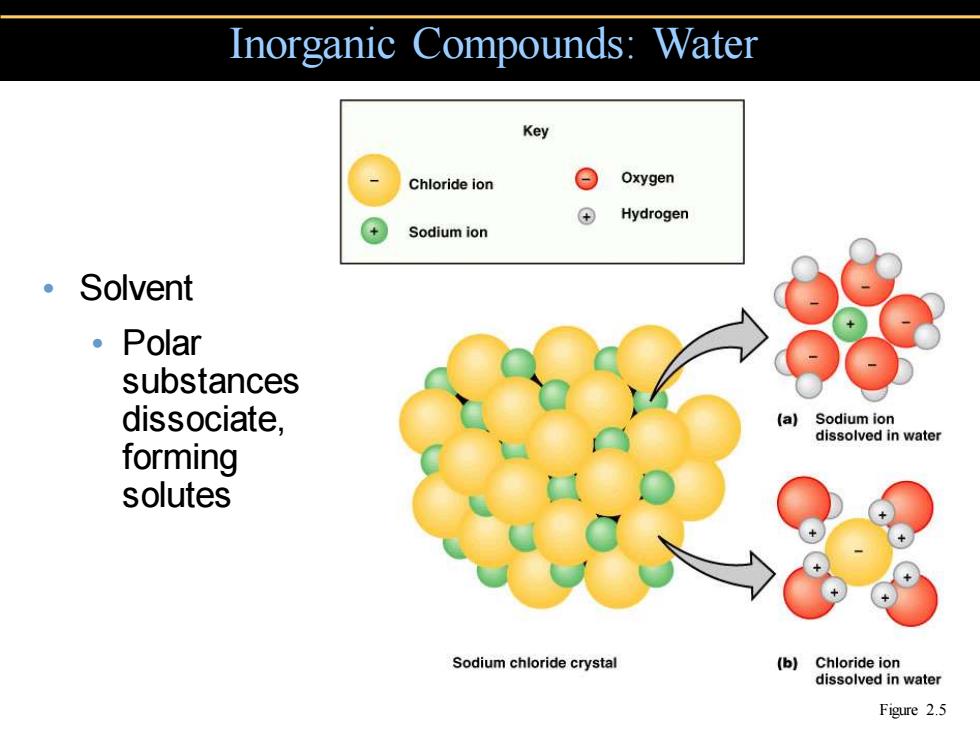
Inorganic Compounds:Water Key Chloride ion g Oxygen ⊕ Hydrogen Sodium ion 。Solvent 。Polar substances dissociate, (a)Sodium ion dissolved in water forming solutes Sodium chloride crystal (b)Chloride ion dissolved in water Figure 2.5
• Solvent • Polar substances dissociate, forming solutes Inorganic Compounds: Water Figure 2.5
Inorganic Compounds:Water H+and OH-participate in chemical reactions
• H+ and OH− participate in chemical reactions Inorganic Compounds: Water