
4.4.1Additionalreaction4.4.1.1Catalytic HydrogenationH2R-HC=CH-RR-C=CRPt或 Pd,Ni△H=-175.4 kJ/molHR-CHCHRPt或Pd,Ni△H=-136.9kJ/mo1
4.4.1 Additional reaction 4.4.1.1 Catalytic Hydrogenation △H=-175.4 kJ/mol R C C R' R HC CH R' Pt 或 Pd , Ni H2 R CH2 CH2 R' Pt 或 Pd , Ni H2 △H=-136.9kJ/mol
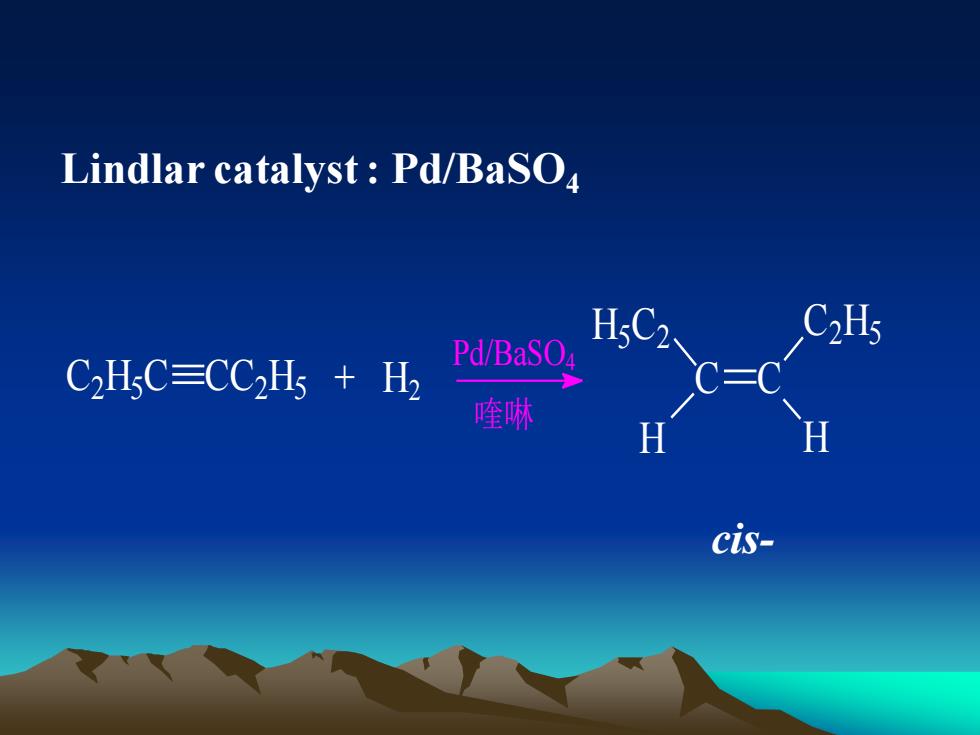
Lindlarcatalyst:Pd/BaSOC2HsH5C2Pd/BaSO4CHsC=CCHs + H喹啉HHcis-
Lindlar catalyst : Pd/BaSO4 C2 H5 C CC2 H5 + H2 Pd/BaSO4 喹啉 C C H5 C2 C2 H5 H H cis-
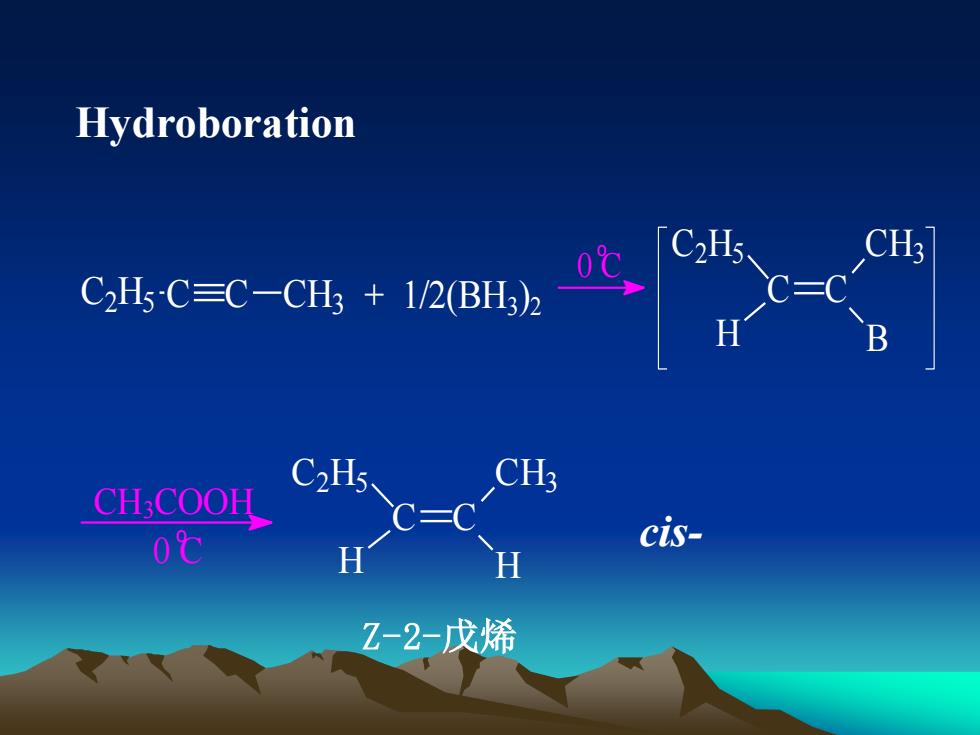
HydroborationCH3CHsC,H5-C=C-CH3 + 1/2(BH3)2一BCH3C2H5CHCOOHcis-0℃HHZ-2-戊烯
Hydroboration + 1/2(BH3 ) C2 H5 C C CH3 2 o 0 C C C C2 H5 CH3 H B o 0 C CH3COOH C C C2 H5 CH3 H H Z-2-戊烯 cis-
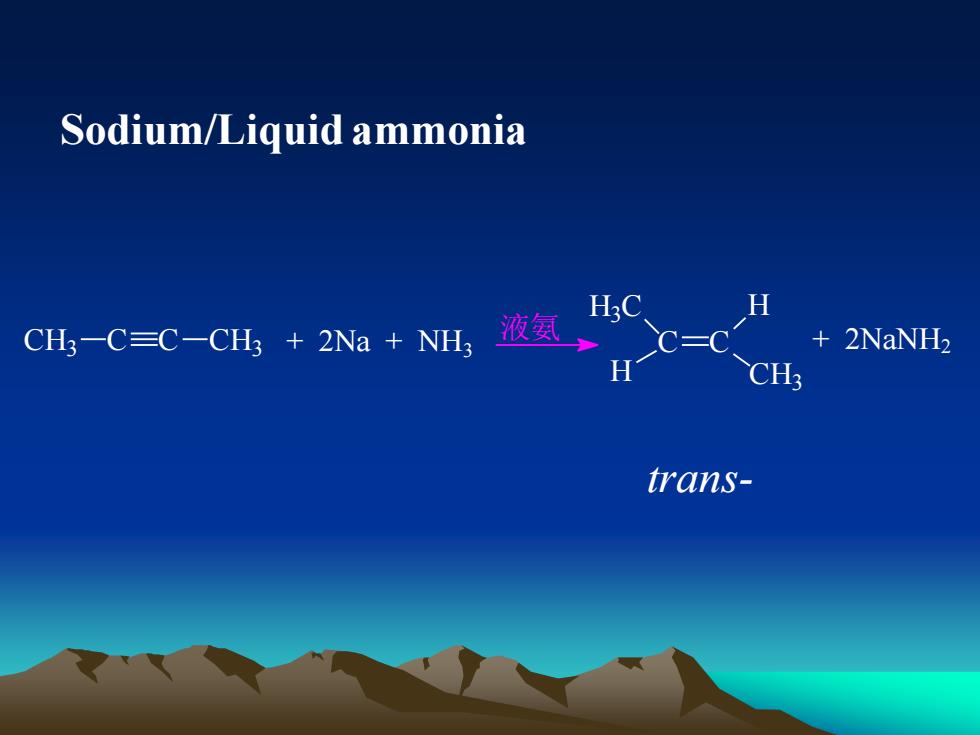
Sodium/Liquid ammoniaHH3C液氨+2NaNH2CH-C=C-CH3 + 2Na +NHHCH3trans-
Sodium/Liquid ammonia CH3 C C CH3 + 2Na + NH3 液氨 C C + 2NaNH2 H H CH3 H3 C trans-

MechanismR-C=C-R + Na-→ Na + R-C=C-R游离基负离子RR-C=C-R+ NH-三NH2HR乙烯基游离基
Mechanism R C C R + Na Na + + R C C R - 游离基负离子 - R C C R + NH3 NH2 - + C C R H R 乙烯基游离基