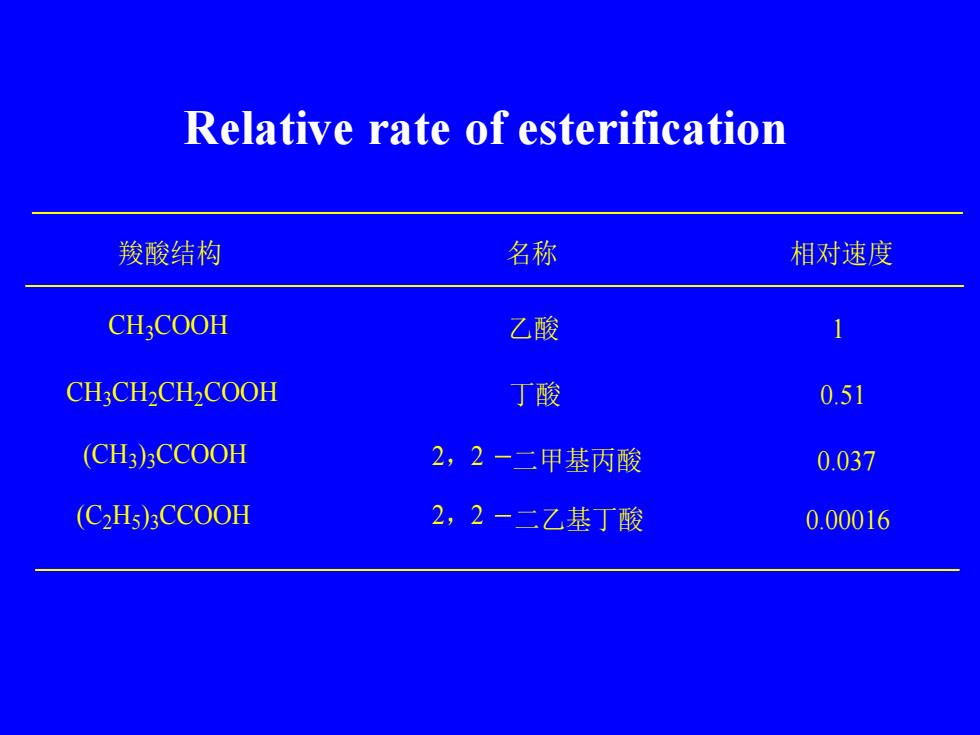
Relative rate of esterification名称羧酸结构相对速度CHCOOH乙酸CH,CH,CH,COOH丁酸0.51(CH3)3CCOOH2,2-二甲基丙酸0.0372,2-二乙基丁酸(C2Hs)3CCOOH0.00016
Relative rate of esterification CH3COOH CH3CH2CH2COOH (CH3 ) 3CCOOH (C2H5) 3CCOOH 羧酸结构 名称 相对速度 乙酸 1 丁酸 0.51 2,2 二甲基丙酸 0.037 2,2 二乙基丁酸 0.00016
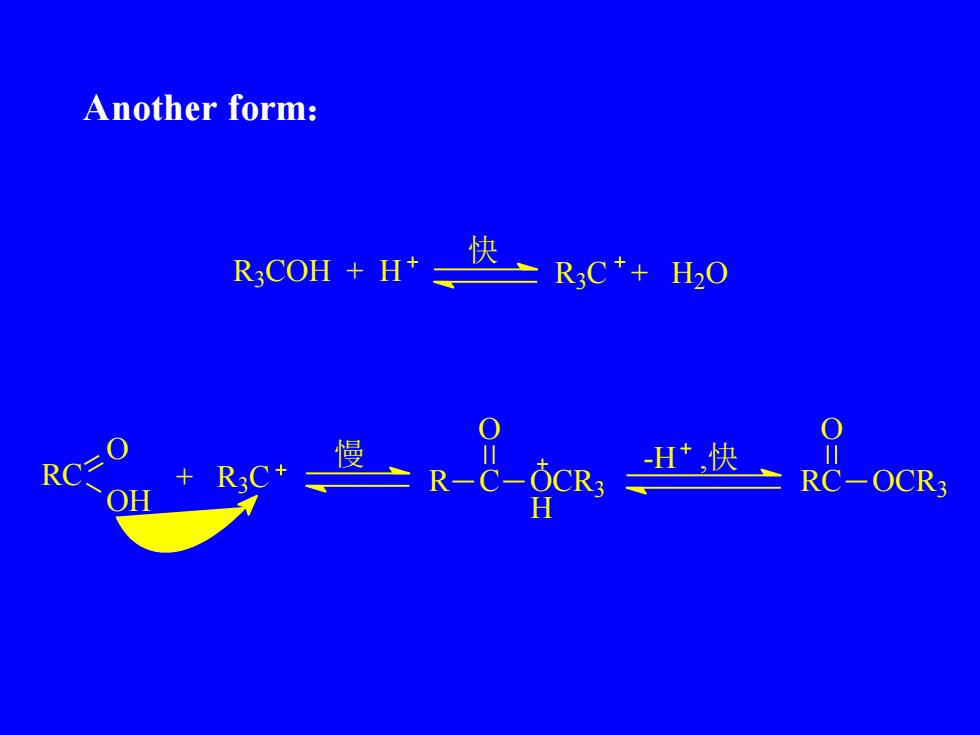
Anotherform:快R,COH + H+R,C++H.0OO慢川川-H,快RC=R3C+0CR3+RCRC-0CR3OHH
Another form: R3 COH + H R3 C + H2O 快 RC O OH + R3 C 慢 R C O OCR3 H -H ,快 RC OCR3 O
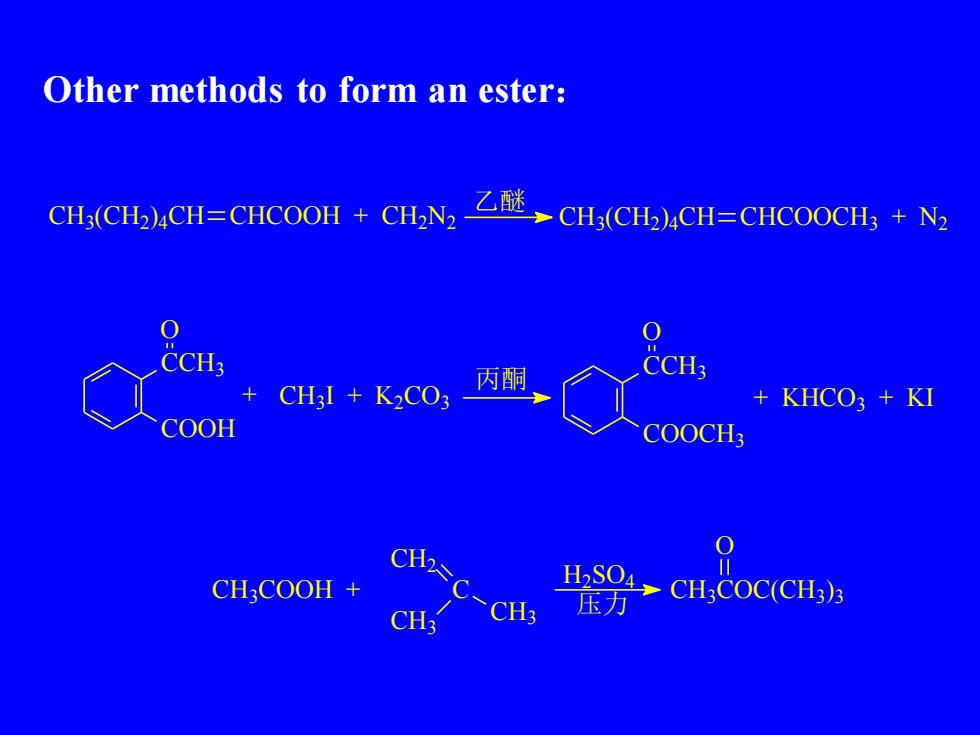
Other methods to form an ester:乙醚CHNCH3(CH2)4CH=CHCOOH+#CH3(CH2)4CH=CHCOOCH3+N20O=CCH3CCH3丙酮XCH,I + K,CO3KHCO3+KI+COOHCOOCH30CH2H2SO4CH:COOHC1CH,COC(CH3)3+压力CH3CH3
Other methods to form an ester: CH3 (CH2 ) 4CH CHCOOH + CH2N2 乙醚 CH3 (CH2 ) 4CH CHCOOCH3 + N2 CCH3 COOH O + CH3I + K2CO3 丙酮 CCH3 COOCH3 O + KHCO3 + KI CH3COOH + C CH2 CH3 CH3 H2 SO4 压力 CH3COC(CH3 ) 3 O
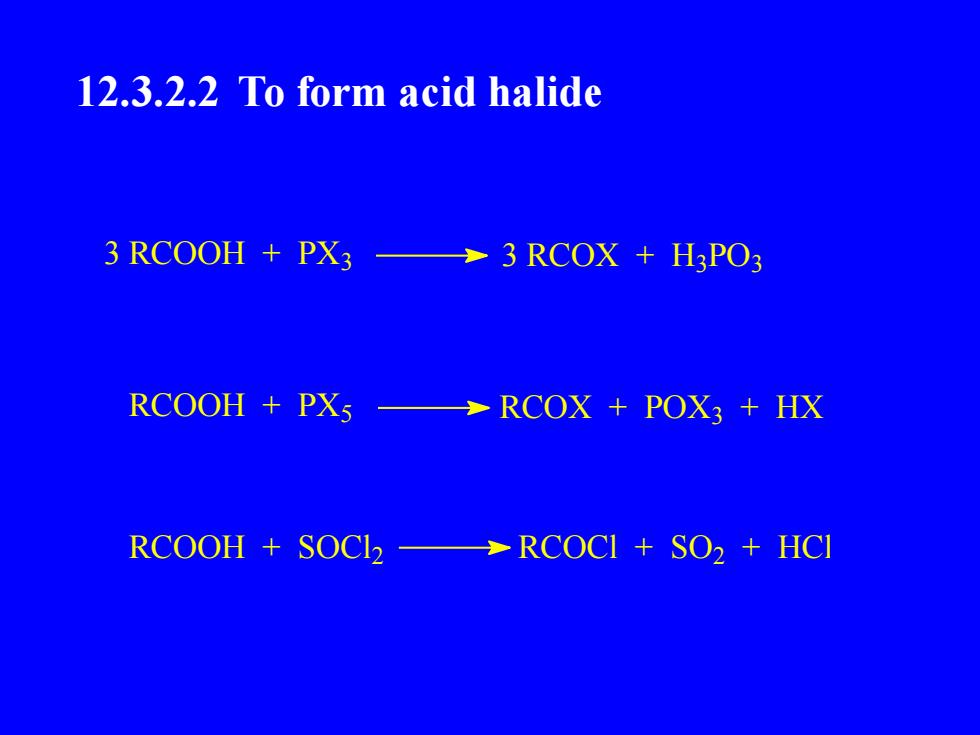
12.3.2.2 To form acid halide3 RCOOH + PX33 RCOX + H3PO3RCOOH + PXsRCOX + POX3 + HXRCOOH + SOCl2RCOCI + SO2 + HCI
12.3.2.2 To form acid halide 3 RCOOH + PX3 3 RCOX + H3 PO3 RCOOH + PX5 RCOX + POX3 + HX RCOOH + SOCl 2 RCOCl + SO2 + HCl
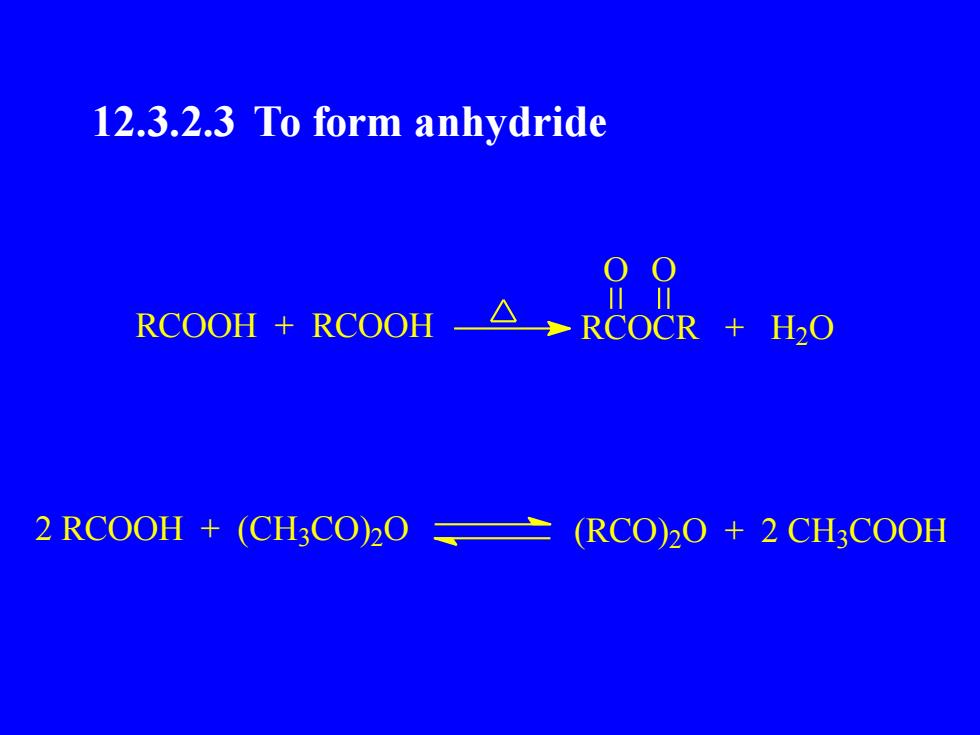
12.3.2.3 To form anhydride001川L+ RCOOHRCOOHRCOCRH20+2RCOOH +(CH3CO)2O(RCO)2O + 2 CH3COOH
12.3.2.3 To form anhydride RCOOH + RCOOH RCOCR + H2 O O O 2 RCOOH + (CH3 CO) 2 O (RCO) 2 O + 2 CH3 COOH