
20 TYPES OF POLYMERS 2.3.1 Linear If a polymer is built from strictly difunctional monomers,the result is a linear polymer chain.A scale model of a typical linear polymer molecule made from 0.5 cm diameter clothesline rope would be about 3 m long.This is not a bad analogy:the chains are long, flexible,and essentially one-dimensional structures.The term linear can be somewhat misleading,however,because the molecules do not necessarily assume a geometrically linear conformation as shown in the figure.Some of the better analogies for what these macromolecules look like is a bowl of spaghetti or tangled strands of yarn.The nomenclature for homopolymers usually simply puts "poly"before the name of the repeat unit,which is particularly simple for most addition polymers,such as in polystyrene.If the repeat unit has more than one word,parentheses are used to indicate what is repeated,such as in poly(vinyl chloride).Many condensation polymers would have fairly complex names using this rule,so most are simply referred to by their class (e.g..a polyester)or by their trade names (Dacron,Nylon,Lexan.etc.). 2.3.1.1 Random Copolymers Polymers consisting of chains that contain a single repeating unit are known as homopolymers(this includes many polymers made by addition and condensation polymerization).If,however,the chains contain a random arrangement of two separate and distinct repeating units,the polymer is known as a random or statistical copolymer,or simply copolymer.A random copolymer might be formed by the addition polymerization of a mixture of two different vinyl monomers A and B(the degree of 'randomness"depends on the relative amounts and reactivities of A and B,as we shall see later)and can be represented as AABAAABBABAAB and called poly(A-co-B),where the first repeating unit listed is the one present in the greater amount.For example,a random synthetic rubber copolymer of 75%butadiene and 25% styrene would be termed poly(butadiene-co-styrene).Of course,ter-(3-)and higher multipolymers are possible:nature makes a regular tetrapolymer(RNA and DNA,which are complex polymers with four bases arranged according to genetic codes)and polypeptides that are copolymers with 20 different repeat units (amino acids). It must be emphasized that the products of condensation polymerizations that require two different monomers to provide the necessary functional groups,for example,a diacid and a diamine,are not copolymers because they contain a single repeating unit.If,however, two different diamines were used in the polymerization,there would be two distinct repeating units and this could be correctly termed a copolymer. Example 2.2 Illustrate the repeating units that result when 3 mol of hexamethylene diamine (I)are condensed with 2 mol of adipic acid (II)and I mol of sebacic acid (IID) and name the resulting copolymer H,N(CH2法NH2HO-C (CH2)C-OH HO-C-(CHC-OH ① an Solution.The two repeating units are From ()and (II) From (T)and (II)
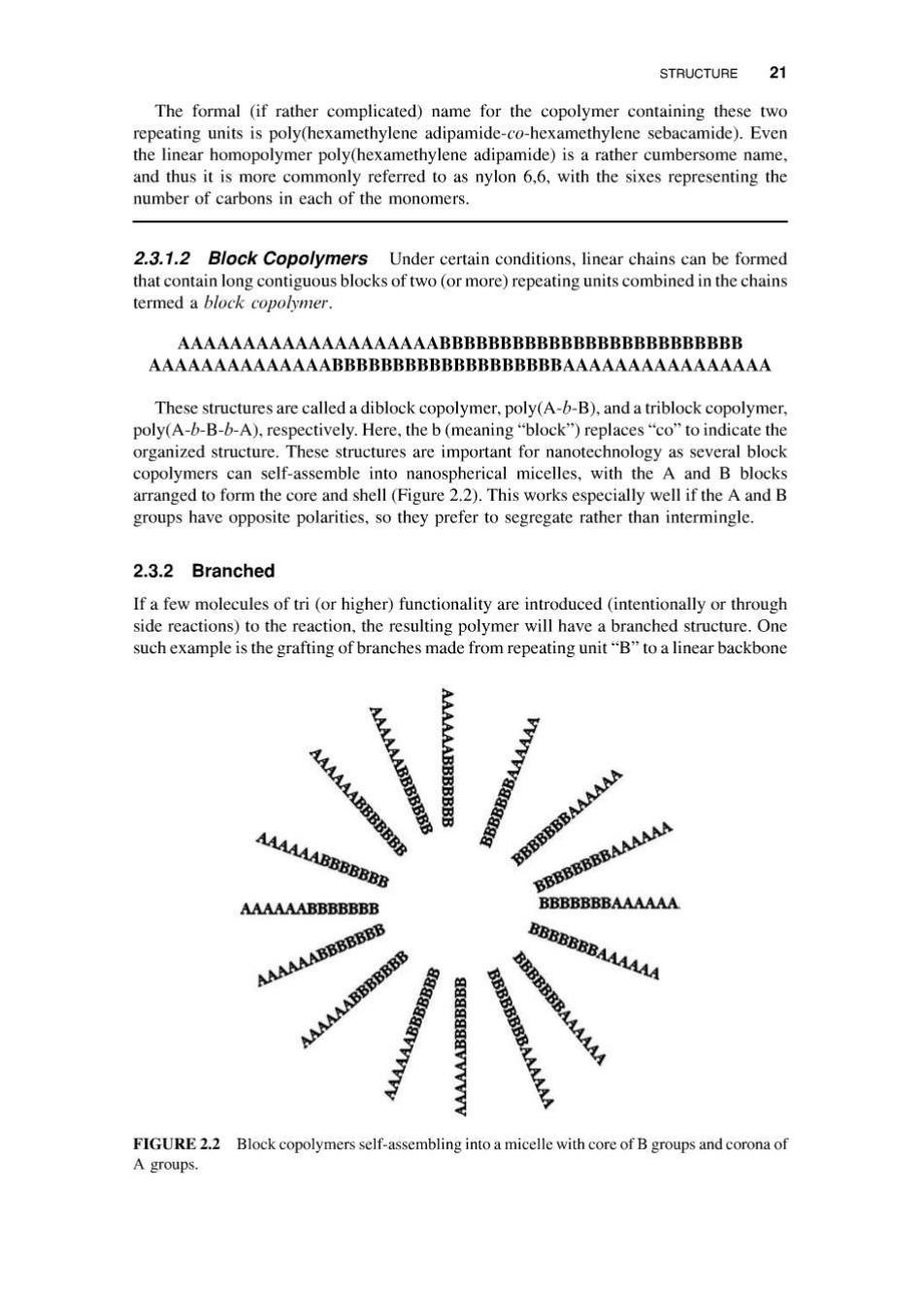
STRUCTURE 21 The formal (if rather complicated)name for the copolymer containing these two repeating units is poly(hexamethylene adipamide-co-hexamethylene sebacamide).Even the linear homopolymer poly(hexamethylene adipamide)is a rather cumbersome name, and thus it is more commonly referred to as nylon 6,6,with the sixes representing the number of carbons in each of the monomers. 2.3.1.2 Block Copolymers Under certain conditions,linear chains can be formed that contain long contiguous blocks of two(or more)repeating units combined in the chains termed a block copolymer. AAAAAAAAAAAAAAAAAAAABBBBBBBBBBBBBBBBBBBBBBBBB AAAAAAAAAAAAAABBBBBBBBBBBBBBBBBBBAAAAAAAAAAAAAAAA These structures are called a diblock copolymer,poly(A-b-B),and a triblock copolymer, poly(A-b-B-b-A).respectively.Here,the b(meaning"block")replaces"co"to indicate the organized structure.These structures are important for nanotechnology as several block copolymers can self-assemble into nanospherical micelles,with the A and B blocks arranged to form the core and shell(Figure 2.2).This works especially well if the A and B groups have opposite polarities.so they prefer to segregate rather than intermingle. 2.3.2 Branched If a few molecules of tri(or higher)functionality are introduced(intentionally or through side reactions)to the reaction,the resulting polymer will have a branched structure.One such example is the grafting of branches made from repeating unit"B"to a linear backbone AAAAAA AAABBBBBBB AAAAAABBBBBBB 图 BBBB BBBBBBBAAAAAA AAAAAABBBBBBB BBBBBBBAAAAAA BBBBBBBBAAAAAA AAAAAABBBBBBB BBBBBBBAAAAAA AAAAAABBBBBBB AAAAAABBBBBBB BBBBBBBAAAAAA AAAAAABBBBBBB BBBBBB y AAAAA FIGURE 2.2 Block copolymers self-assembling into a micelle with core of B groups and corona of A groups
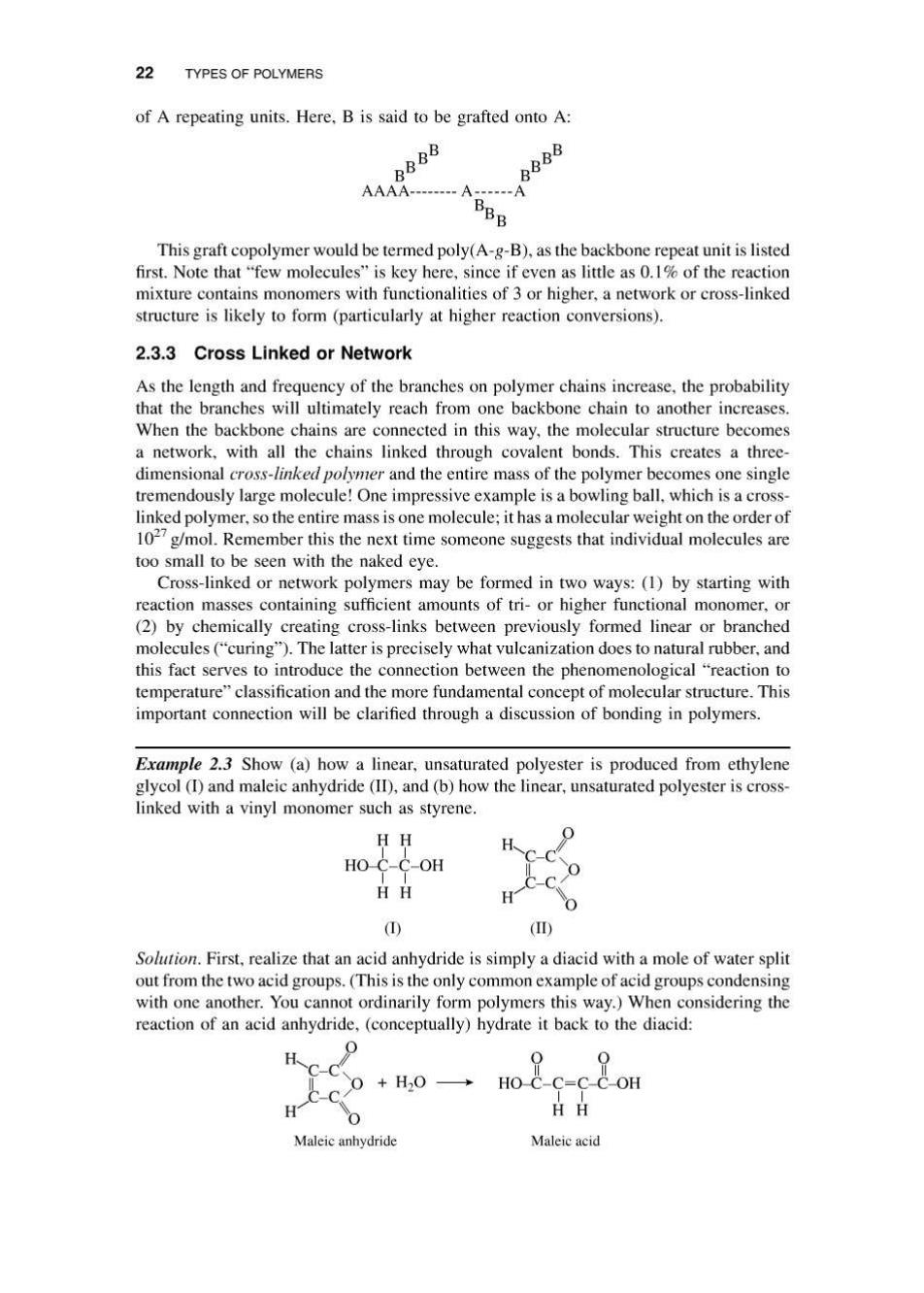
22 TYPES OF POLYMERS of A repeating units.Here,B is said to be grafted onto A: BBBB 888 AAAA--------A------A This graft copolymer would be termed poly(A-g-B),as the backbone repeat unit is listed first.Note that "few molecules"is key here,since if even as little as 0.1%of the reaction mixture contains monomers with functionalities of 3 or higher,a network or cross-linked structure is likely to form (particularly at higher reaction conversions). 2.3.3 Cross Linked or Network As the length and frequency of the branches on polymer chains increase,the probability that the branches will ultimately reach from one backbone chain to another increases. When the backbone chains are connected in this way,the molecular structure becomes a network,with all the chains linked through covalent bonds.This creates a three- dimensional cross-linked polymer and the entire mass of the polymer becomes one single tremendously large molecule!One impressive example is a bowling ball,which is a cross- linked polymer,so the entire mass is one molecule:it has a molecular weight on the order of 1027g/mol.Remember this the next time someone suggests that individual molecules are too small to be seen with the naked eye. Cross-linked or network polymers may be formed in two ways:(1)by starting with reaction masses containing sufficient amounts of tri-or higher functional monomer,or (2)by chemically creating cross-links between previously formed linear or branched molecules("curing").The latter is precisely what vulcanization does to natural rubber,and this fact serves to introduce the connection between the phenomenological "reaction to temperature"classification and the more fundamental concept of molecular structure.This important connection will be clarified through a discussion of bonding in polymers. Example 2.3 Show (a)how a linear,unsaturated polyester is produced from ethylene glycol (I)and maleic anhydride (ID),and(b)how the linear,unsaturated polyester is cross- linked with a vinyl monomer such as styrene. HH HO C-C-OH HH ① () Solution.First,realize that an acid anhydride is simply a diacid with a mole of water split out from the two acid groups.(This is the only common example of acid groups condensing with one another.You cannot ordinarily form polymers this way.)When considering the reaction of an acid anhydride,(conceptually)hydrate it back to the diacid: 0 H CC、 0+H20→ HO C-C=C-C-OH C-C H- Maleic anhydride Maleic acid
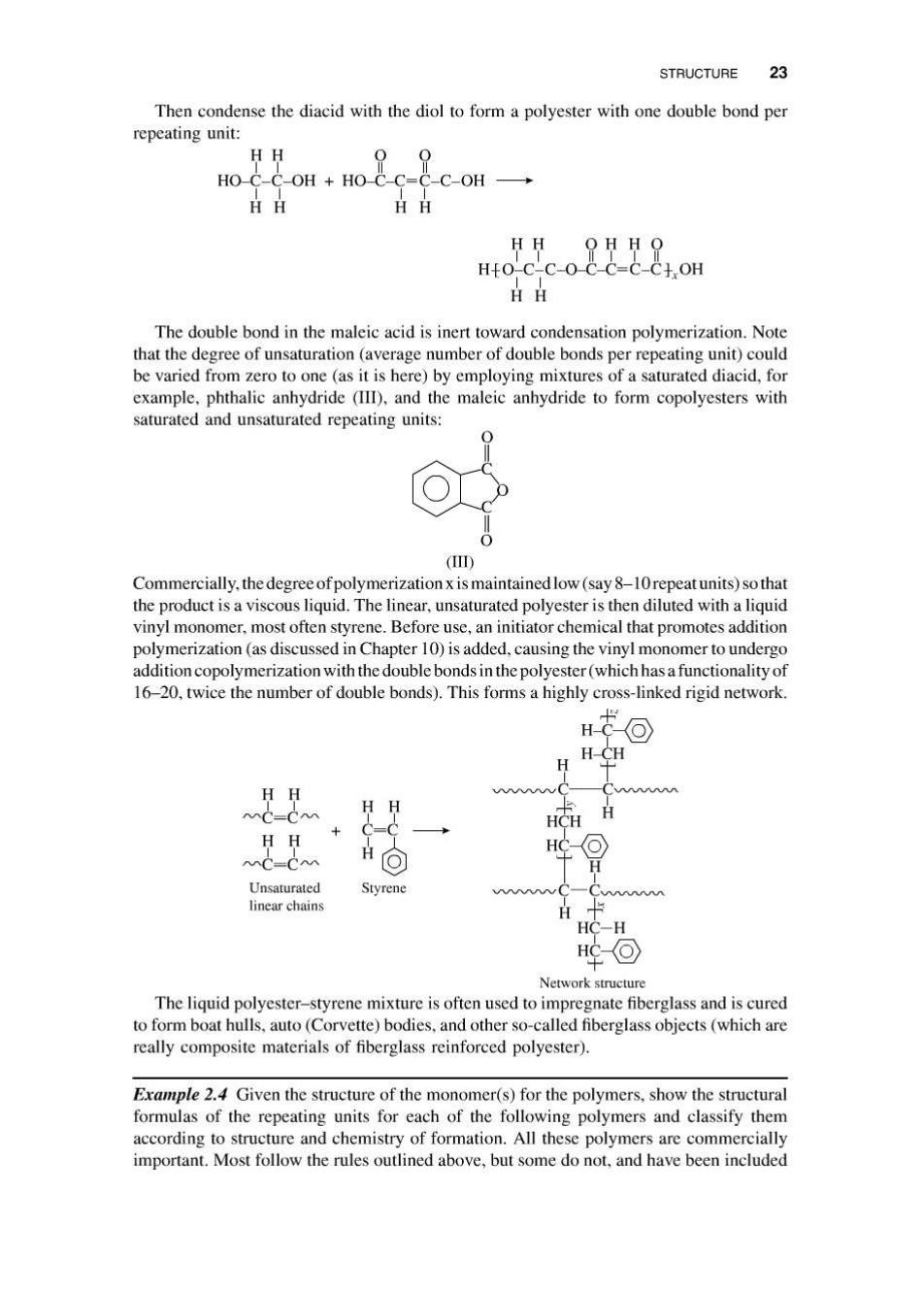
STRUCTURE 23 Then condense the diacid with the diol to form a polyester with one double bond per repeating unit: HH 0 HO C-C-OH HO-C-C=C-C-OH- HH HH oco8是8om HH The double bond in the maleic acid is inert toward condensation polymerization.Note that the degree of unsaturation(average number of double bonds per repeating unit)could be varied from zero to one(as it is here)by employing mixtures of a saturated diacid,for example,phthalic anhydride (III),and the maleic anhydride to form copolyesters with saturated and unsaturated repeating units: (I) Commercially.the degree ofpolymerization x is maintained low(say 8-10repeat units)so that the product is a viscous liquid.The linear,unsaturated polyester is then diluted with a liquid vinyl monomer,most often styrene.Before use,an initiator chemical that promotes addition polymerization(as discussed in Chapter 10)is added,causing the vinyl monomer to undergo addition copolymerization with the double bonds in the polyester(which has a functionality of 16-20,twice the number of double bonds).This forms a highly cross-linked rigid network. H-CC H-CH HH H HCH HH MC=CM H HC H Unsaturated Styrene wwwc-Cw linear chains HC-H 华回 Network structure The liquid polyester-styrene mixture is often used to impregnate fiberglass and is cured to form boat hulls,auto(Corvette)bodies,and other so-called fiberglass objects(which are really composite materials of fiberglass reinforced polyester). Example 2.4 Given the structure of the monomer(s)for the polymers,show the structural formulas of the repeating units for each of the following polymers and classify them according to structure and chemistry of formation.All these polymers are commercially important.Most follow the rules outlined above,but some do not,and have been included
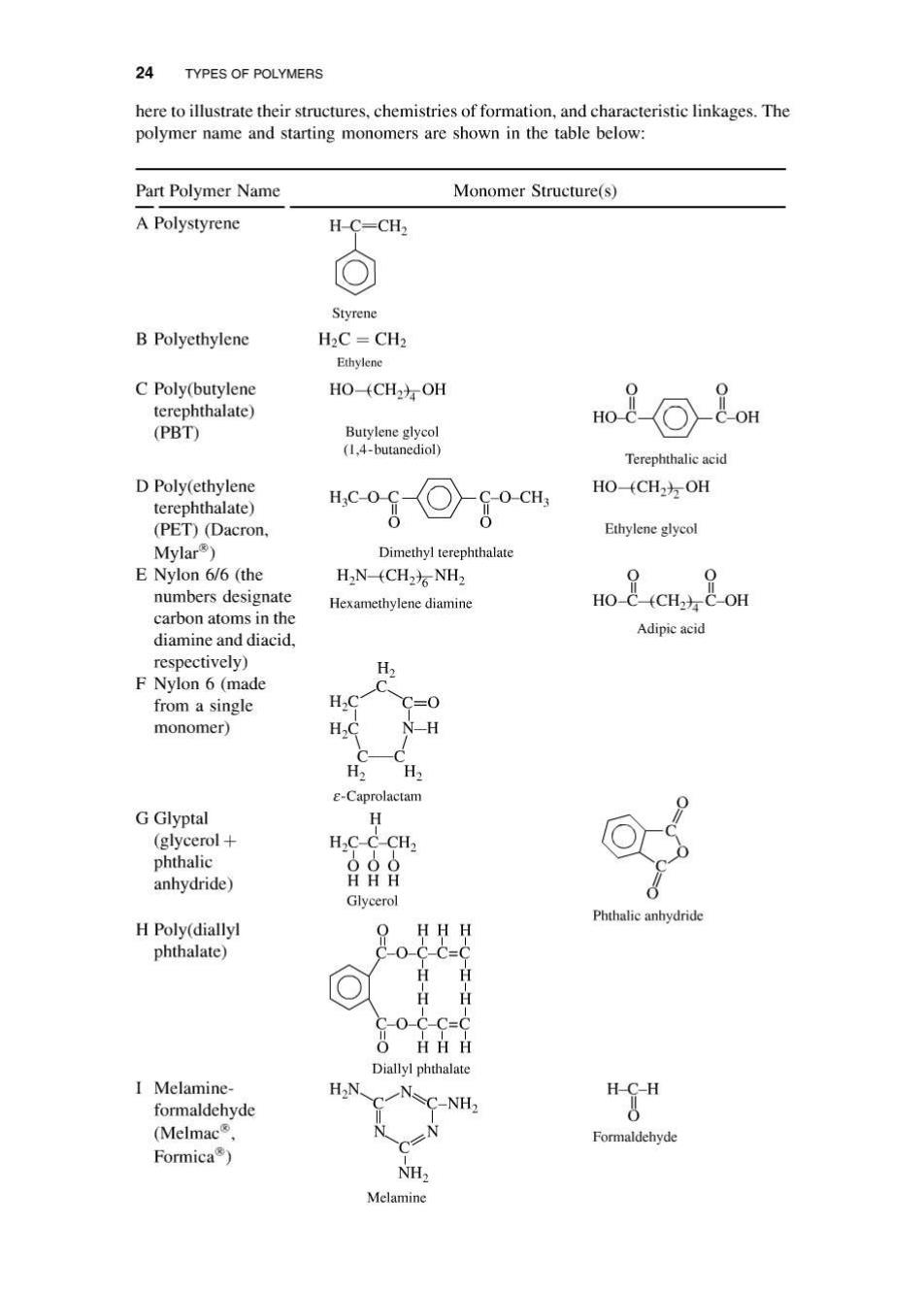
24 TYPES OF POLYMERS here to illustrate their structures,chemistries of formation,and characteristic linkages.The polymer name and starting monomers are shown in the table below: Part Polymer Name Monomer Structure(s) A Polystyrene H-C=CH2 Styrene B Polyethylene H2C CH2 Ethylene C Poly(butylene HO(CH2方OH terephthalate) (PBT) Butylene glycol (1.4-butanediol) Terephthalic acid D Poly(ethylene terephthalate) HC-O o-cH. HO(CH2方OH (PET)(Dacron, Ethylene glycol Mylar) Dimethyl terephthalate E Nylon 6/6(the H2N-(CH2)NH2 numbers designate Hexamethylene diamine carbon atoms in the Adipic acid diamine and diacid, respectively) F Nylon 6(made from a single H monomer) H E-Caprolactam G Glyptal H (glycerol+ H2C-C-CH2 phthalic 000 anhydride) HHH Glycerol Phthalic anhydride H Poly(diallyl HH H phthalate) 80-Cc= 日日 HH O-C-C=C OH日日 Diallyl phthalate I Melamine- formaldehyde C-NH2 (Melmac Formaldehyde Formica) NH2 Melamine