
CHEMISTRY OF SYNTHESIS 15 polyimide,the carboxylic acid groups must be close together.Of course,this forms only a single bond.To form a polyimide,there needs to be another diacid as part of the R functional group and another amine in the R2 group. 2.2.1.4 Polyurethanes Polyurethanes are made by the reaction of a molecule with two isocyanate groups (a diisocyanate)and two alcohols (a diol).In this reaction,the hydrogen from the alcohol is transferred to the nitrogen atom of the isocyanate group,and a new bond is formed between the isocyanate carbon and the alcohol oxygen. OH HO-R2-OH +O=C=N-R-N=C=0 HO-(-R-0-C-N-R2-)-N=C=0 Diol Diisocyanate Polyurethane As observed for the polyesters,polyamides,and polyimides,polyurethanes also have a characteristic bond,-(O-C-N)-,in their backbone (with the carbon having a =O)that makes these polymers easily identifiable.Note that after the reaction to form a urethane bond,the end groups(an alcohol and an isocyanate)are still available to grow the polymer chain further. 2.2.1.5 Polycarbonates One additional group of condensation polymers highly important in industry is polycarbonates.Polycarbonates are a special subcase of polyesters, in that one monomer has only a single carbon,resulting in the characteristic linkage: 9 0-C0 A common reaction to produce polycarbonates involves a diol (bisphenol-A)and phosgene,which is a diacid chloride(with functionality similar to a dicarboxylic acid)(see part N in Example 2.4). 2.2.1.6 Monomers with Tri-(or Higher)Functionality The above examples are for polymers formed from difunctional reactants.If either reactant has only one functional group,no polymer can result.However,monomers with functionalities higher than two are also used to make polymers.For example,glycerin is a trifunctional molecule (three alcohol groups)that can be used in polyesterification.Molecules with three or more functional groups tend to make network (or cross-linked)polymers,as the reaction can proceed to form a three-dimensional network instead of just a linear polymer as difunctional monomers do. HHH H-C-C-C-H 0OO H日H 2.2.1.7 Monomers with Two Different Functional Groups It is possible that the starting molecule for a condensation polymerization has two (or more)different functional groups and does not require the presence of two different monomers.Two
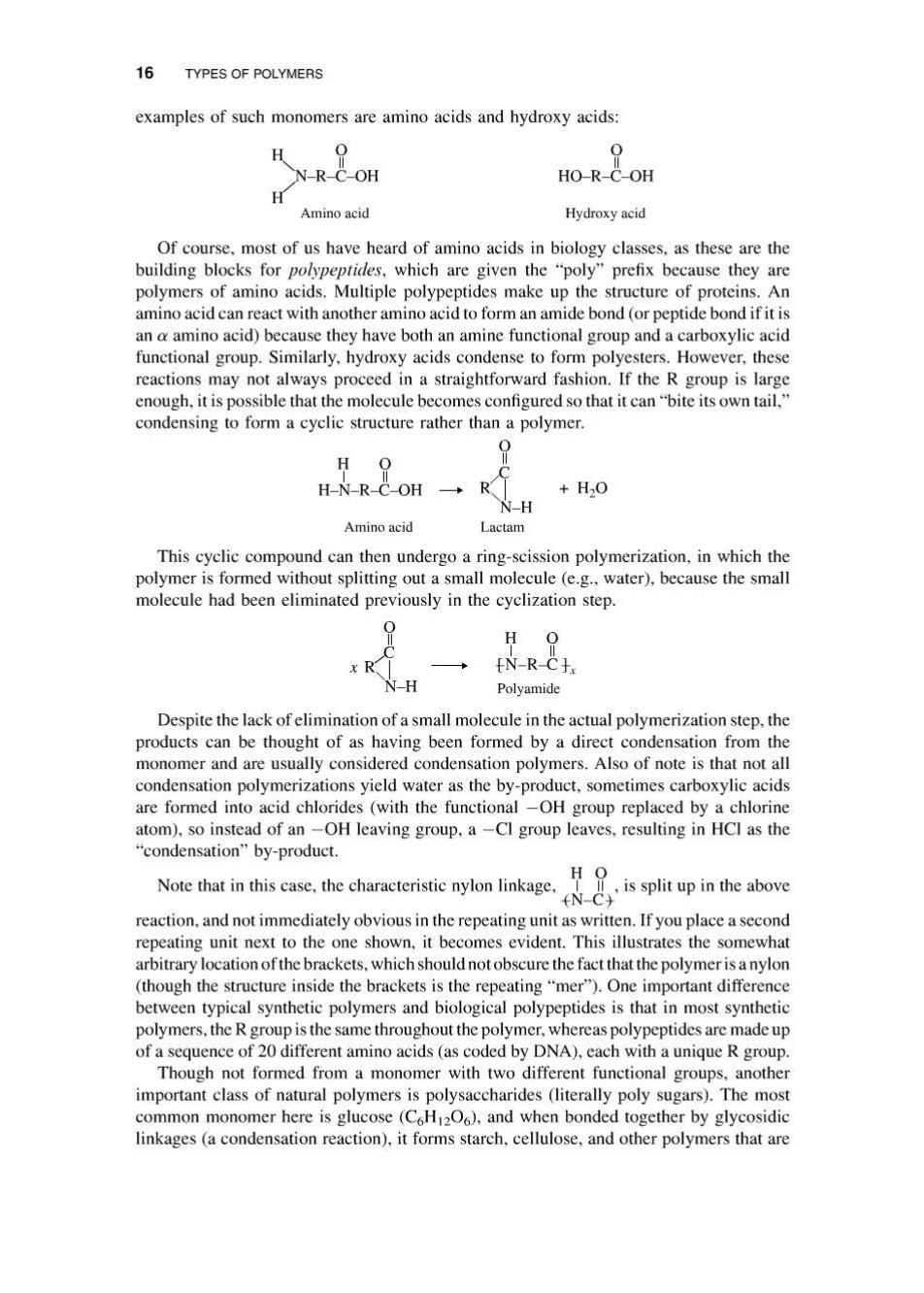
16 TYPES OF POLYMERS examples of such monomers are amino acids and hydroxy acids: H 0 0 N-R-C-OH HO-R-C-OH H Amino acid Hydroxy acid Of course,most of us have heard of amino acids in biology classes,as these are the building blocks for polypeptides,which are given the "poly"prefix because they are polymers of amino acids.Multiple polypeptides make up the structure of proteins.An amino acid can react with another amino acid to form an amide bond (or peptide bond if it is an a amino acid)because they have both an amine functional group and a carboxylic acid functional group.Similarly,hydroxy acids condense to form polyesters.However,these reactions may not always proceed in a straightforward fashion.If the R group is large enough,it is possible that the molecule becomes configured so that it can"bite its own tail," condensing to form a cyclic structure rather than a polymer. H 0 H-N-R-C-OH +H2O N-H Amino acid Lactam This cyclic compound can then undergo a ring-scission polymerization,in which the polymer is formed without splitting out a small molecule (e.g.,water),because the small molecule had been eliminated previously in the cyclization step. H O N-RC于. N-H Polyamide Despite the lack of elimination of a small molecule in the actual polymerization step,the products can be thought of as having been formed by a direct condensation from the monomer and are usually considered condensation polymers.Also of note is that not all condensation polymerizations yield water as the by-product,sometimes carboxylic acids are formed into acid chlorides (with the functional-OH group replaced by a chlorine atom).so instead of an-OH leaving group,a-Cl group leaves,resulting in HCI as the "condensation"by-product. H O Note that in this case,the characteristic nylon linkage,II,is split up in the above N-CY reaction,and not immediately obvious in the repeating unit as written.If you place a second repeating unit next to the one shown,it becomes evident.This illustrates the somewhat arbitrary location of the brackets,which should not obscure the fact that the polymer is a nylon (though the structure inside the brackets is the repeating"mer").One important difference between typical synthetic polymers and biological polypeptides is that in most synthetic polymers,the R group is the same throughout the polymer,whereas polypeptides are made up of a sequence of 20 different amino acids(as coded by DNA),each with a unique R group Though not formed from a monomer with two different functional groups,another important class of natural polymers is polysaccharides (literally poly sugars).The most common monomer here is glucose (C6H1206),and when bonded together by glycosidic linkages(a condensation reaction),it forms starch,cellulose,and other polymers that are

CHEMISTRY OF SYNTHESIS 17 important for structural rigidity (particularly in plants)and energy storage in living organisms,as most starches can be broken down(depolymerized)easily. 2.2.2 Addition Polymerization The second polymer formation reaction is known as addition polymerization and its products as addition (or chain or free radical)polymers.Addition polymerizations have two distinct characteristics: 1.No molecule is split out;hence,the repeat unit has the same chemical formula as the monomer. 2.The polymerization reaction involves the opening of a double bond. Monomers of the general type C=C undergo addition polymerization as e--tch The double bond "opens up,"forming bonds to other monomers at each end,so a carbon-carbon double bond is difunctional according to our general definition.(Note that a monomer with two double bonds has a functionality of four;this type of monomer is often referred to as a cross-linking agent as it results in the formation of network structures.)The question of what happens at the ends of addition polymers will be deferred until we reach Chapter 9 on polymerization mechanisms.Also,note that carbon-oxygen double bonds, while common in monomers and polymers,are stable and unreactive for addition polymerization. An important subclass of the double bond containing monomers is the vinyl monomer: H H X Addition polymerization is occasionally referred to as vinyl polymerization.Table 2.1 lists some commercially important vinyl monomers,with different substituents replacing X. TABLE 2.1 Some Commercially Important Vinyl Monomers Monomer -X Ethylene H Styrene Vinyl chloride -CI Propylene -CH3 Acrylonitrile -CEN Although aromatic rings are often symbolized by.this is a poor representation of resonance-stabilized structures,which are completely inert to addition polymerization.They are more properly symbolized by to avoid confusion with the ordinary double bond.To save space in this book,we will sometimes also use to represent the aromatic (or phenyl)ring

18 TYPES OF POLYMERS Example 2.I Lactic acid can be dehydrated to form acrylic acid according to the following reaction: HC O H HO HOCC-OH→ C=C-O-OH +H2O H H Lactic acid Acrylic acid Both these acids can be used as monomers for polymerization.Write the structural formulas for the repeating units for each of the polymers that would be formed if starting from (A)lactic acid and (B)acrylic acid. Solution.Lactic acid is a hydroxy acid having-OH and-COOH functional groups.It will form a condensation polymer by splitting out water to give HC Q HtO-C-CHOH H As this is a monomer with two different functional groups,the structure in brackets splits up the characteristic ester linkage Acrylic acid is a vinyl monomer with a double bond(since it has only one carboxylic acid, it would not be useful in a condensation polymerization).It undergoes addition polymeri- zation to form HO +C-CH H C-O OH Interestingly,the ester linkage in poly(lactic acid),PLA,is easily hydrolyzed,breaking down the polymer.Thus,this polymer has found applications in biodegradable plastics for products ranging from food packaging to tissue engineering scaffolds for medical applications.Poly(acrylic acid),or PAA,is not degradable,but the pendant carboxylic acid group makes this polymer extremely hydrophilic and thus it is used as a superabsor- bent(particularly important in diapers). Although most molecules with two C=C bonds (dialkenes or simply dienes)have a functionality of 4 for addition polymerization,in conjugated dienes,the two double bonds are separated by only one single C-C bond,having a functionality of 2,so they form linear polymers.A conjugated diene is a unique monomer for polymerization because (unlike molecules where the double bonds that act as cross-linking agents are further separated)the double bonds here act in the same way as a single double bond in vinyl polymerizations. ccc-c 1234 The addition polymerization of a conjugated diene results in an unsaturated polymer,or a polymer that contains double bonds,either in the backbone of the polymer or in the pendant group.Furthermore,conjugated diene polymerizations can result in several
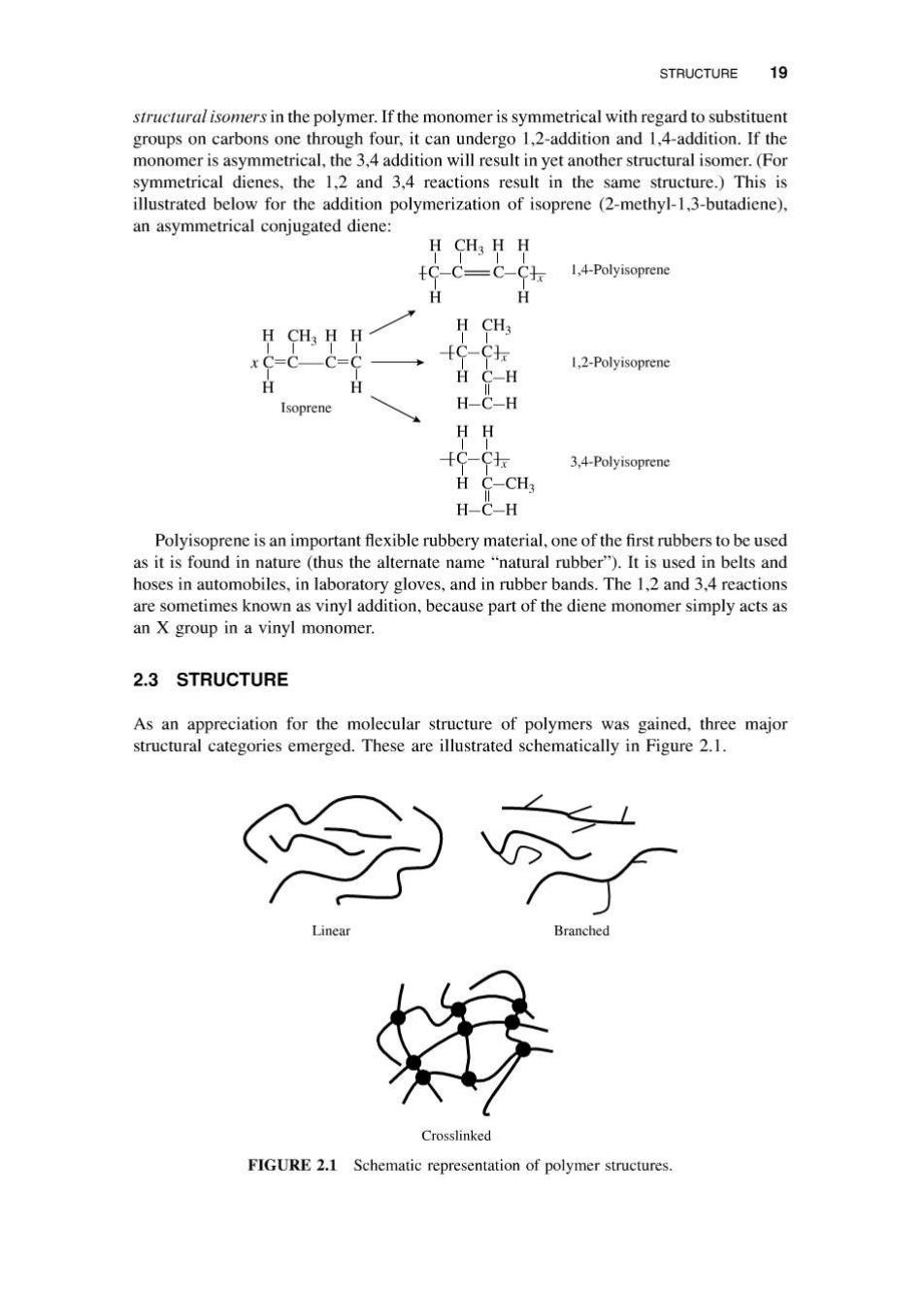
STRUCTURE 19 structural isomers in the polymer.If the monomer is symmetrical with regard to substituent groups on carbons one through four,it can undergo 1,2-addition and 1,4-addition.If the monomer is asymmetrical,the 3,4 addition will result in yet another structural isomer.(For symmetrical dienes,the 1,2 and 3.4 reactions result in the same structure.)This is illustrated below for the addition polymerization of isoprene (2-methyl-1,3-butadiene). an asymmetrical conjugated diene: H CH3 HH c-C-c 1,4-Polyisoprene H CH3 HH H CH3 -C=C C-CI 1.2-Polyisoprene H C-H Isoprene H-C-H HH IC-CI 3.4-Polyisoprene H C-CH H-C-H Polyisoprene is an important flexible rubbery material,one of the first rubbers to be used as it is found in nature (thus the alternate name "natural rubber").It is used in belts and hoses in automobiles,in laboratory gloves,and in rubber bands.The 1,2 and 3.4 reactions are sometimes known as vinyl addition,because part of the diene monomer simply acts as an X group in a vinyl monomer. 2.3 STRUCTURE As an appreciation for the molecular structure of polymers was gained,three major structural categories emerged.These are illustrated schematically in Figure 2.1. Linear Branched Crosslinked FIGURE 2.1 Schematic representation of polymer structures