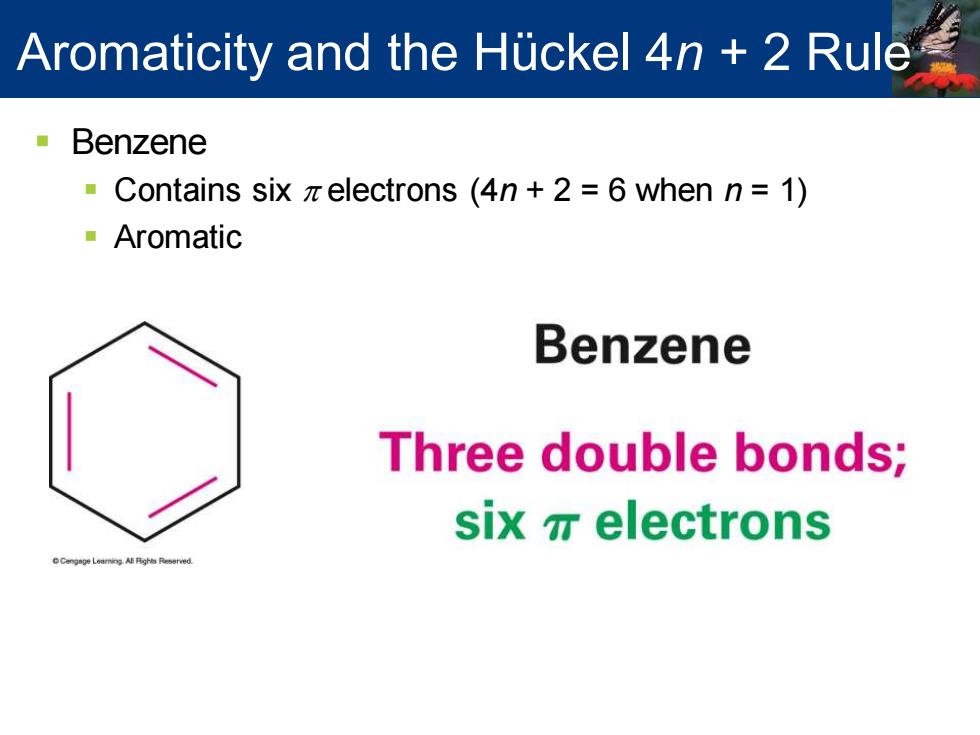
Aromaticity and the Huckel 4n 2 Rule Benzene Contains six zelectrons (4n+2 6 when n=1) Aromatic Benzene Three double bonds; six electrons
▪ Benzene ▪ Contains six p electrons (4n + 2 = 6 when n = 1) ▪ Aromatic Aromaticity and the Hückel 4n + 2 Rule
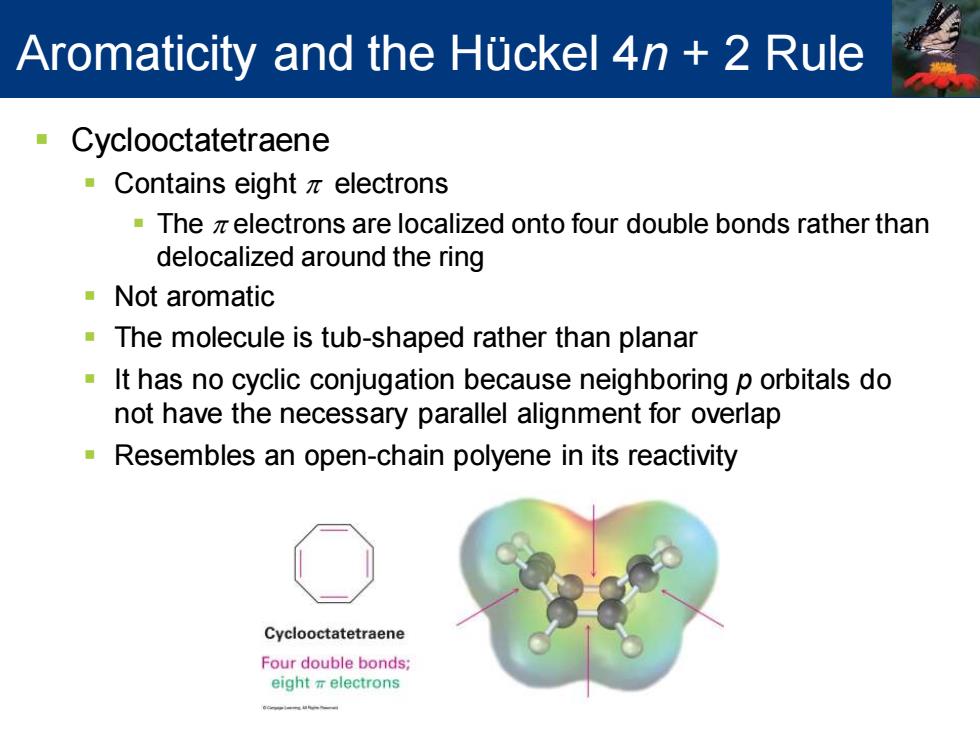
Aromaticity and the Huckel 4n 2 Rule Cyclooctatetraene Contains eight z electrons The z electrons are localized onto four double bonds rather than delocalized around the ring Not aromatic The molecule is tub-shaped rather than planar It has no cyclic conjugation because neighboring p orbitals do not have the necessary parallel alignment for overlap Resembles an open-chain polyene in its reactivity Cyclooctatetraene Four double bonds; eight electrons
▪ Cyclooctatetraene ▪ Contains eight p electrons ▪ The p electrons are localized onto four double bonds rather than delocalized around the ring ▪ Not aromatic ▪ The molecule is tub-shaped rather than planar ▪ It has no cyclic conjugation because neighboring p orbitals do not have the necessary parallel alignment for overlap ▪ Resembles an open-chain polyene in its reactivity Aromaticity and the Hückel 4n + 2 Rule
Aromaticity and the Huckel 4n 2 Rule Energy Levels of Cyclic Conjugated Molecules (4n 2 Electrons) There is always a single lowest-lying MO,above which the MOs come in degenerate pairs -When electrons fill the various molecular orbitals,one pair of electrons fills the lowest-lying orbital and two pairs of electrons fill each of the n successive energy levels -a total of 4n 2.Any other number would leave a bonding energy level partially unfilled
Energy Levels of Cyclic Conjugated Molecules (4n + 2 Electrons) ▪ There is always a single lowest-lying MO, above which the MOs come in degenerate pairs ▪ When electrons fill the various molecular orbitals, one pair of electrons fills the lowest-lying orbital and two pairs of electrons fill each of the n successive energy levels – a total of 4n + 2. Any other number would leave a bonding energy level partially unfilled Aromaticity and the Hückel 4n + 2 Rule
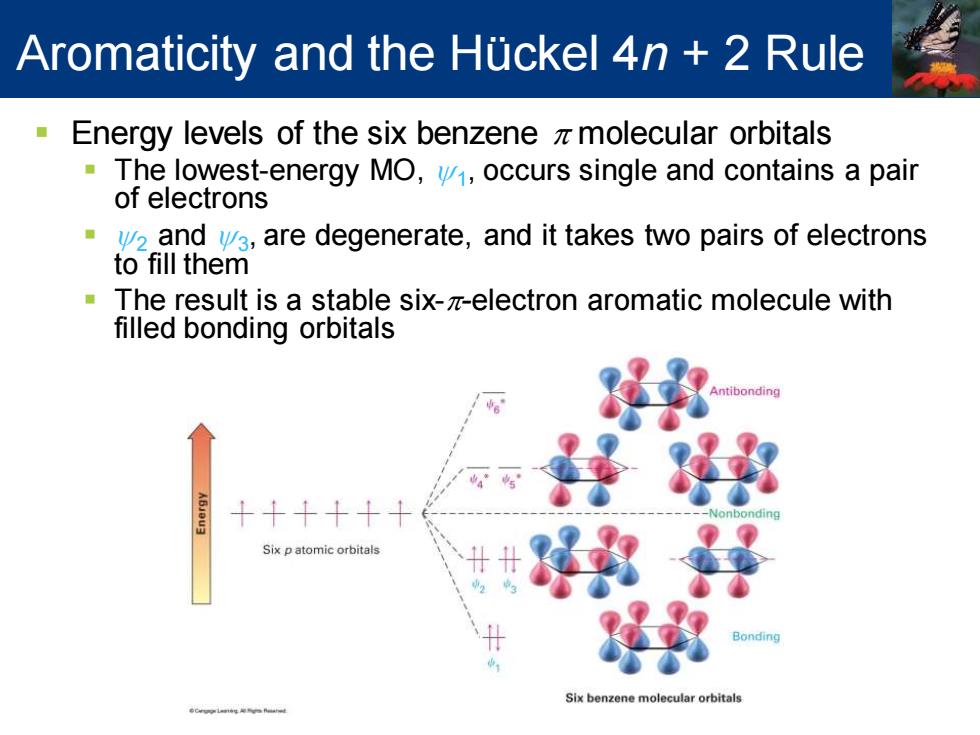
Aromaticity and the Huckel 4n 2 Rule Energy levels of the six benzene z molecular orbitals The lowest-energy MO,v,occurs single and contains a pair of electrons 2 and ya,are degenerate,and it takes two pairs of electrons to fill them The result is a stable six-z-electron aromatic molecule with filled bonding orbitals onding Six p atomic orbitals ondin Six benzene molecular orbitals DCRPLaneg NVRa
▪ Energy levels of the six benzene p molecular orbitals ▪ The lowest-energy MO, y1 , occurs single and contains a pair of electrons ▪ y2 and y3 , are degenerate, and it takes two pairs of electrons to fill them ▪ The result is a stable six-p-electron aromatic molecule with filled bonding orbitals Aromaticity and the Hückel 4n + 2 Rule
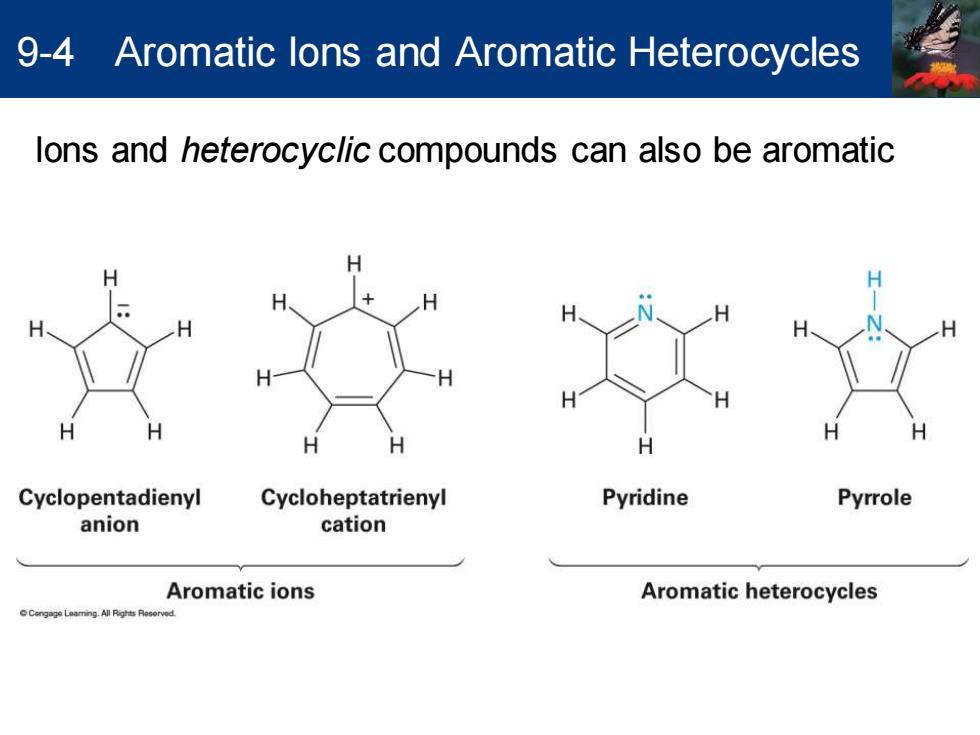
9-4 Aromatic lons and Aromatic Heterocycles lons and heterocyclic compounds can also be aromatic H H Cyclopentadienyl Cycloheptatrienyl Pyridine Pyrrole anion cation Aromatic ions Aromatic heterocycles
Ions and heterocyclic compounds can also be aromatic 9-4 Aromatic Ions and Aromatic Heterocycles