Aromatic lons and Aromatic Heterocycles Aromatic lons -There are three ways in which the hydrogen might be removed from cyclopenta-1,3-diene and cyclohepta-1,3,5- triene The hydrogen can be removed with both electrons (H: leaving a carbocation The hydrogen can be removed with one electron (H.) leaving a carbon radical The hydrogen can be removed with no electrons (H+) leaving a carbon anion,or carbanion
Aromatic Ions ▪ There are three ways in which the hydrogen might be removed from cyclopenta-1,3-diene and cyclohepta-1,3,5- triene ▪ The hydrogen can be removed with both electrons (H:- ) leaving a carbocation ▪ The hydrogen can be removed with one electron (H. ) leaving a carbon radical ▪ The hydrogen can be removed with no electrons (H+ ) leaving a carbon anion, or carbanion Aromatic Ions and Aromatic Heterocycles

Aromatic lons and Aromatic Heterocycles 4n 2 rule predicts cyclopentadienyl anion and cycloheptatrienyl cation to be aromatic HH -H:- or H. or H+ Cyclopenta-1,3-diene Cyclopentadienyl Cyclopentadienyl Cyclopentadienyl cation radical anion (four electrons) (five electrons】 (six electrons】 -H: or H. or H+ Cyclohepta-1,3,5-triene Cycloheptatrienyl Cycloheptatrienyl Cycloheptatrienyl cation radical anion (six m electrons) (seven electrons) (eight a electrons)
4n + 2 rule predicts cyclopentadienyl anion and cycloheptatrienyl cation to be aromatic Aromatic Ions and Aromatic Heterocycles
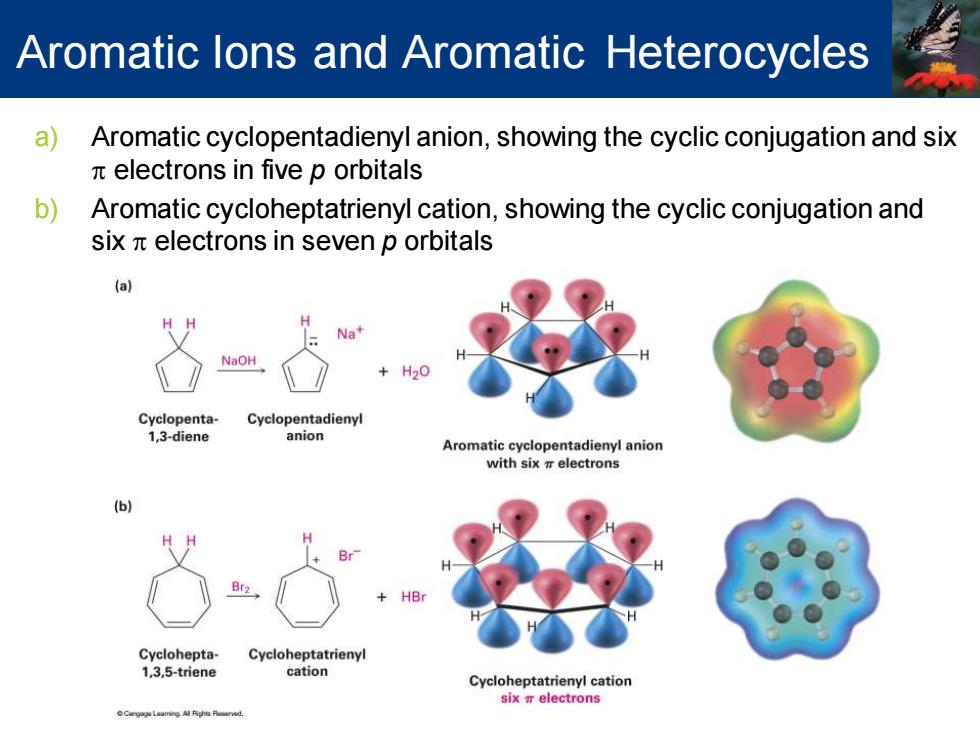
Aromatic lons and Aromatic Heterocycles a Aromatic cyclopentadienyl anion,showing the cyclic conjugation and six electrons in five p orbitals b) Aromatic cycloheptatrienyl cation,showing the cyclic conjugation and six n electrons in seven p orbitals Na+ NaOH Cyclopenta- Cyclopentadienyl 1,3-diene anion Aromatic cyclopentadienyl anion with six electrons (b) HBr Cyclohepta- Cycloheptatrienyl 1,3,5-triene cation Cycloheptatrienyl cation six electrons
Aromatic Ions and Aromatic Heterocycles a) Aromatic cyclopentadienyl anion, showing the cyclic conjugation and six p electrons in five p orbitals b) Aromatic cycloheptatrienyl cation, showing the cyclic conjugation and six p electrons in seven p orbitals
Aromatic lons and Aromatic Heterocycles Aromatic Heterocycles A cyclic compound that contains atoms of two or more different elements in its ring,usually carbon along with nitrogen,oxygen,or sulfur Pyridine is much like benzene in its z electron structure A six-membered heterocycle with nitrogen in its ring Each of the five sp2-hybridized carbons has a p orbital perpendicular to the plane of the ring and each p orbital contains one z electron
Aromatic Heterocycles ▪ A cyclic compound that contains atoms of two or more different elements in its ring, usually carbon along with nitrogen, oxygen, or sulfur ▪ Pyridine is much like benzene in its p electron structure ▪ A six-membered heterocycle with nitrogen in its ring ▪ Each of the five sp2 -hybridized carbons has a p orbital perpendicular to the plane of the ring and each p orbital contains one p electron Aromatic Ions and Aromatic Heterocycles
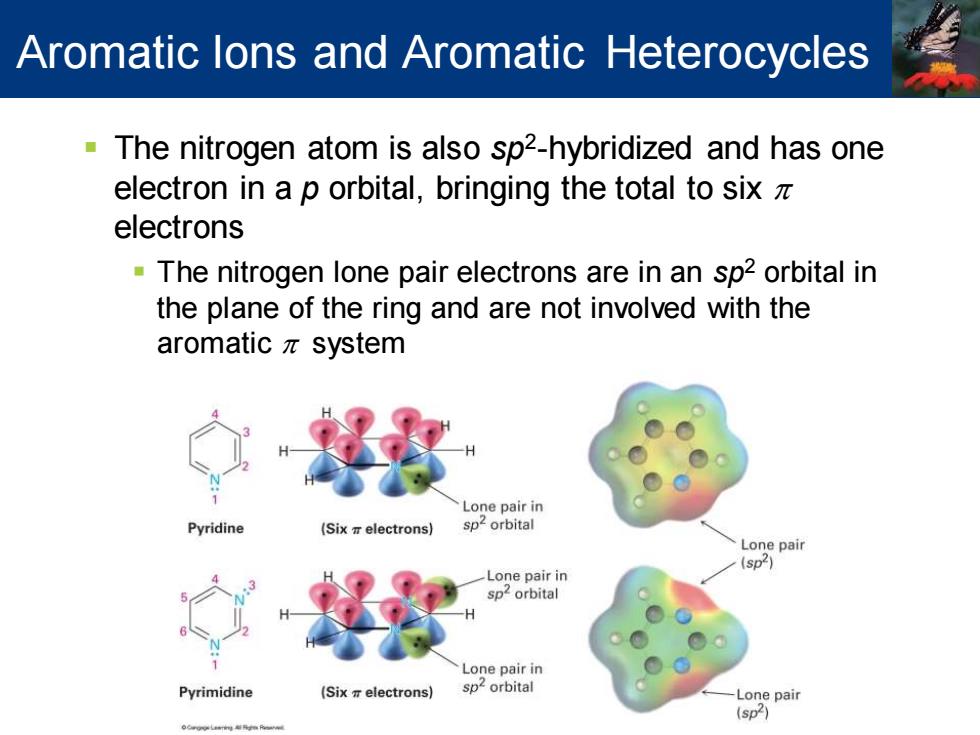
Aromatic lons and Aromatic Heterocycles The nitrogen atom is also sp2-hybridized and has one electron in a p orbital,bringing the total to sixz electrons The nitrogen lone pair electrons are in an sp2 orbital in the plane of the ring and are not involved with the aromaticπsystem Lone pair in Pyridine (Six electrons) Lone pair (sp2) Lone pair in orbital Lone pair in Pyrimidine (Six electrons) sp orbital Lone pair (sp2)
▪ The nitrogen atom is also sp2 -hybridized and has one electron in a p orbital, bringing the total to six p electrons ▪ The nitrogen lone pair electrons are in an sp2 orbital in the plane of the ring and are not involved with the aromatic p system Aromatic Ions and Aromatic Heterocycles