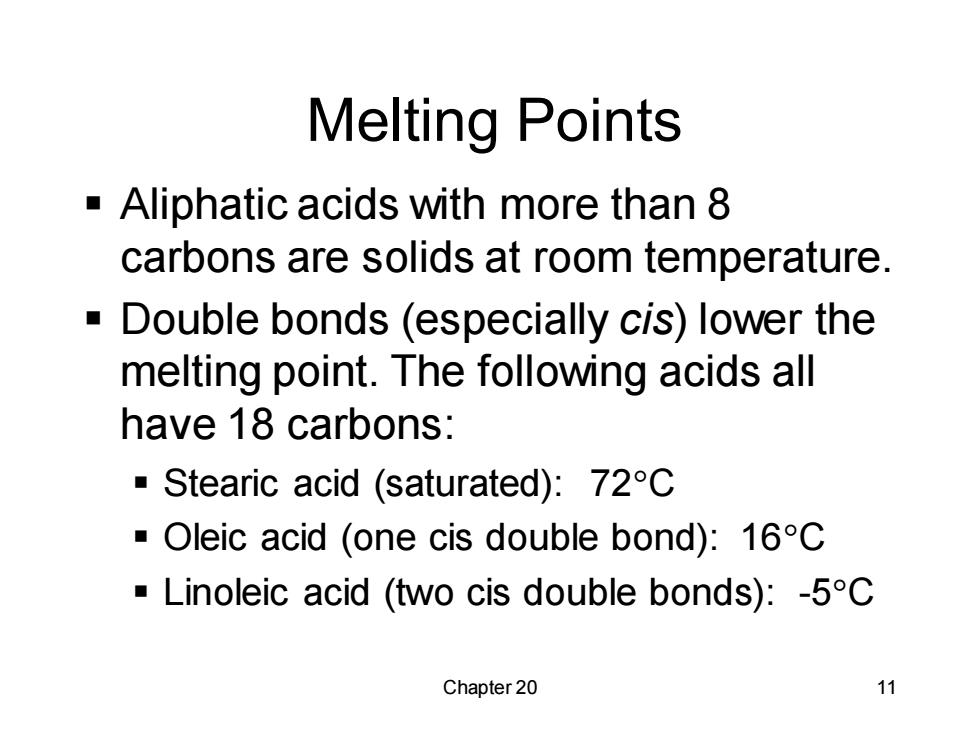
Melting Points Aliphatic acids with more than 8 carbons are solids at room temperature. Double bonds (especially cis)lower the melting point.The following acids all have 18 carbons: Stearic acid (saturated):72C Oleic acid (one cis double bond):16C Linoleic acid (two cis double bonds):-5C Chapter 20 11
Chapter 20 11 Melting Points ▪ Aliphatic acids with more than 8 carbons are solids at room temperature. ▪ Double bonds (especially cis) lower the melting point. The following acids all have 18 carbons: ▪ Stearic acid (saturated): 72C ▪ Oleic acid (one cis double bond): 16C ▪ Linoleic acid (two cis double bonds): -5C

Solubility Water solubility decreases with the length of the carbon chain. With up to 4 carbons,acid is miscible in water. Very soluble in alcohols. Also soluble in relatively nonpolar solvents like chloroform because the hydrogen bonds of the dimer are not disrupted by the nonpolar solvent. Chapter 20 12
Chapter 20 12 Solubility ▪ Water solubility decreases with the length of the carbon chain. ▪ With up to 4 carbons, acid is miscible in water. ▪ Very soluble in alcohols. ▪ Also soluble in relatively nonpolar solvents like chloroform because the hydrogen bonds of the dimer are not disrupted by the nonpolar solvent
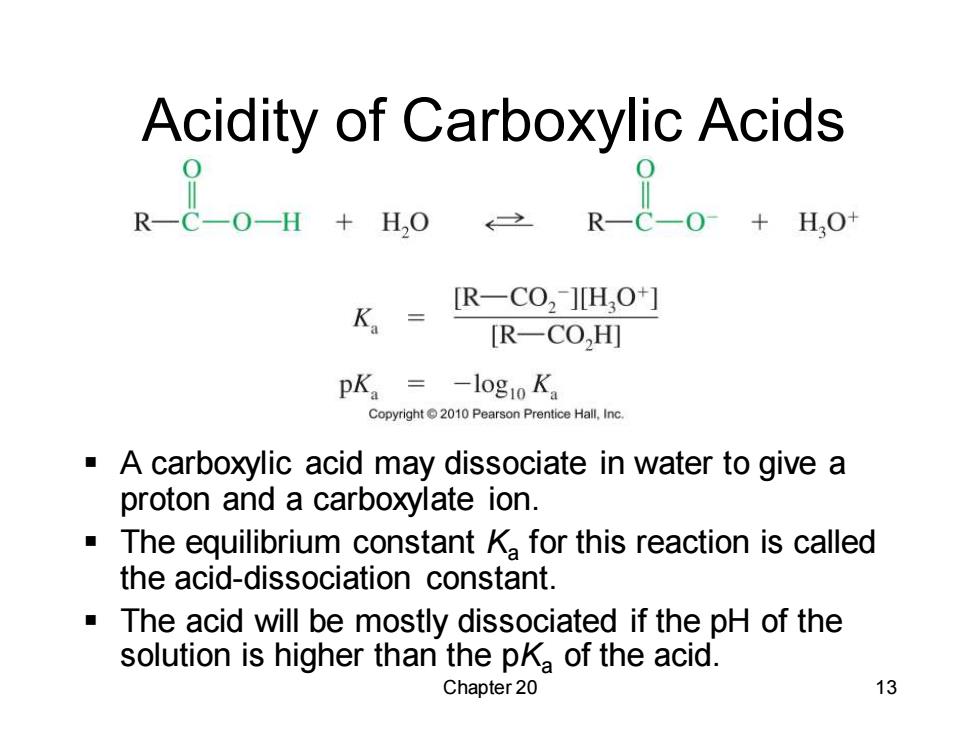
Acidity of Carboxylic Acids 出+点 K= R一CO2]HO+] [R-CO,H] pKa=-log1oK。 Copyright 2010 Pearson Prentice Hall,Inc A carboxylic acid may dissociate in water to give a proton and a carboxylate ion. The equilibrium constant K for this reaction is called the acid-dissociation constant. The acid will be mostly dissociated if the pH of the solution is higher than the pKa of the acid. Chapter 20 13
Chapter 20 13 Acidity of Carboxylic Acids ▪ A carboxylic acid may dissociate in water to give a proton and a carboxylate ion. ▪ The equilibrium constant Ka for this reaction is called the acid-dissociation constant. ▪ The acid will be mostly dissociated if the pH of the solution is higher than the pKa of the acid
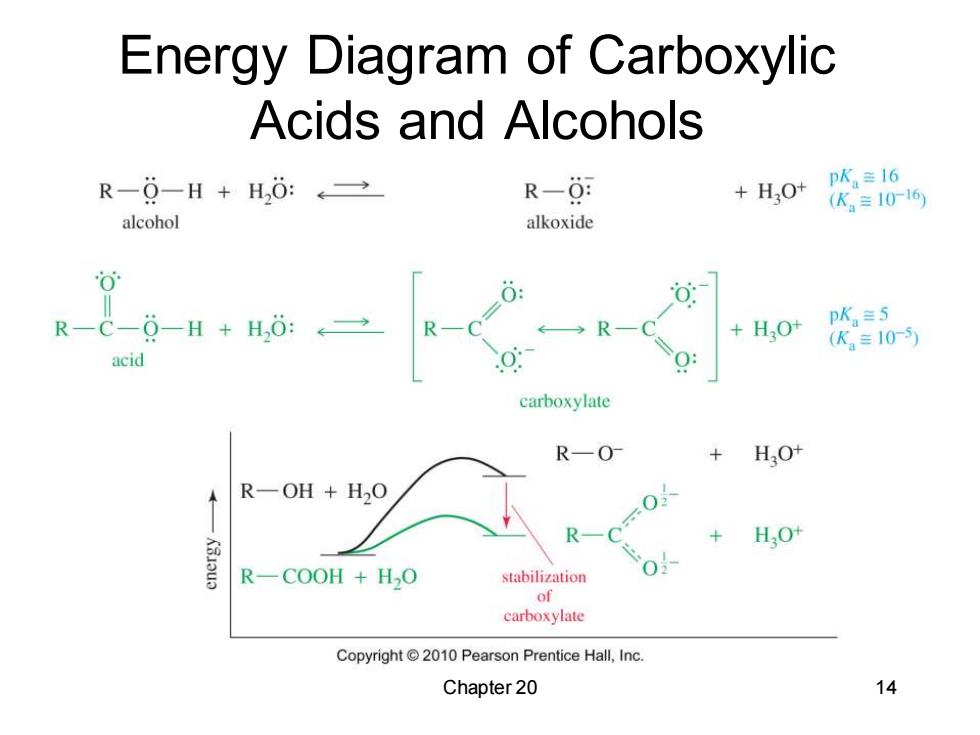
Energy Diagram of Carboxylic Acids and Alcohols R-0-H+H,0:←→ R-0 +H,0+ pK=16 (K=10-16 alcohol alkoxide R-C-0-H+H,0:2 ←→R +HO+ pK=5 (K2=10-5 acid carboxylate R-0- H3O+ R一OH+H2O R HO+ R-COOH +H2O stabilization 0 of carboxylate Copyright2010 Pearson Prentice Hall,Inc. Chapter 20 14
Chapter 20 14 Energy Diagram of Carboxylic Acids and Alcohols
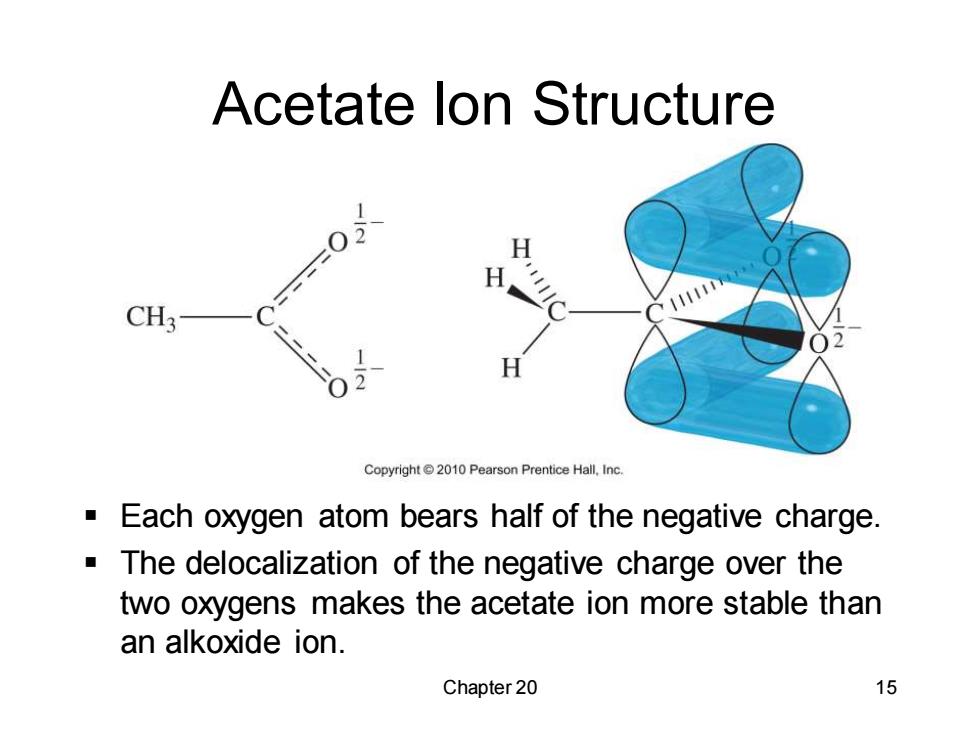
Acetate lon Structure H H CH3 H Copyright 2010 Pearson Prentice Hall,Inc. Each oxygen atom bears half of the negative charge. The delocalization of the negative charge over the two oxygens makes the acetate ion more stable than an alkoxide ion. Chapter 20 15
Chapter 20 15 Acetate Ion Structure ▪ Each oxygen atom bears half of the negative charge. ▪ The delocalization of the negative charge over the two oxygens makes the acetate ion more stable than an alkoxide ion