
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 12 Infrared Spectroscopy and Mass Spectrometry Copyright 2010 Pearson Education,Inc
Chapter 12 Copyright © 2010 Pearson Education, Inc. Organic Chemistry, 7th Edition L. G. Wade, Jr. Infrared Spectroscopy and Mass Spectrometry

Introduction ● Spectroscopy is a technique used to determine the structure of a compound. Most techniques are nondestructive (it destroys little or no sample). Absorption spectroscopy measures the amount of light absorbed by the sample as a function of wavelength. Chapter 12 2
Chapter 12 2 Introduction • Spectroscopy is a technique used to determine the structure of a compound. • Most techniques are nondestructive (it destroys little or no sample). • Absorption spectroscopy measures the amount of light absorbed by the sample as a function of wavelength
Types of Spectroscopy Infrared(IR)spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. Mass spectrometry (MS)fragments the molecule and measures their mass.MS can give the molecular weight of the compound and functional groups. Nuclear magnetic resonance (NMR)spectroscopy analyzes the environment of the hydrogens in a compound.This gives useful clues as to the alkyl and other functional groups present. Ultraviolet (UV)spectroscopy uses electronic transitions to determine bonding patterns. Chapter 12 3
Chapter 12 3 Types of Spectroscopy • Infrared (IR) spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. • Mass spectrometry (MS) fragments the molecule and measures their mass. MS can give the molecular weight of the compound and functional groups. • Nuclear magnetic resonance (NMR) spectroscopy analyzes the environment of the hydrogens in a compound. This gives useful clues as to the alkyl and other functional groups present. • Ultraviolet (UV) spectroscopy uses electronic transitions to determine bonding patterns
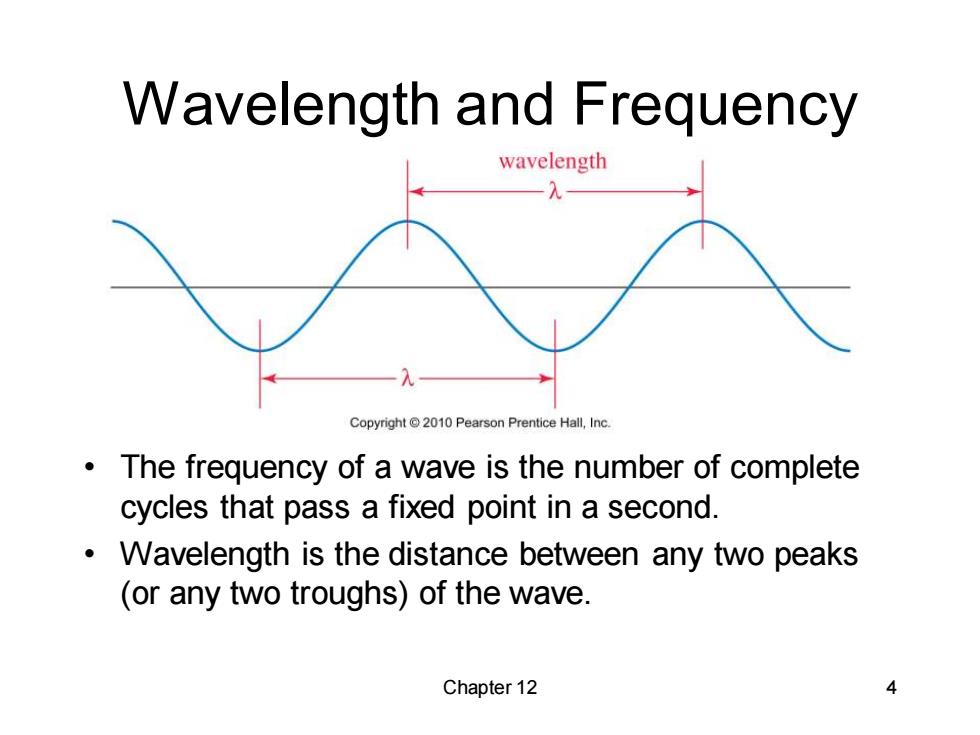
Wavelength and Frequency wavelength Copyright2010 Pearson Prentice Hall,Inc. The frequency of a wave is the number of complete cycles that pass a fixed point in a second. Wavelength is the distance between any two peaks (or any two troughs)of the wave. Chapter 12 4
Chapter 12 4 Wavelength and Frequency • The frequency of a wave is the number of complete cycles that pass a fixed point in a second. • Wavelength is the distance between any two peaks (or any two troughs) of the wave
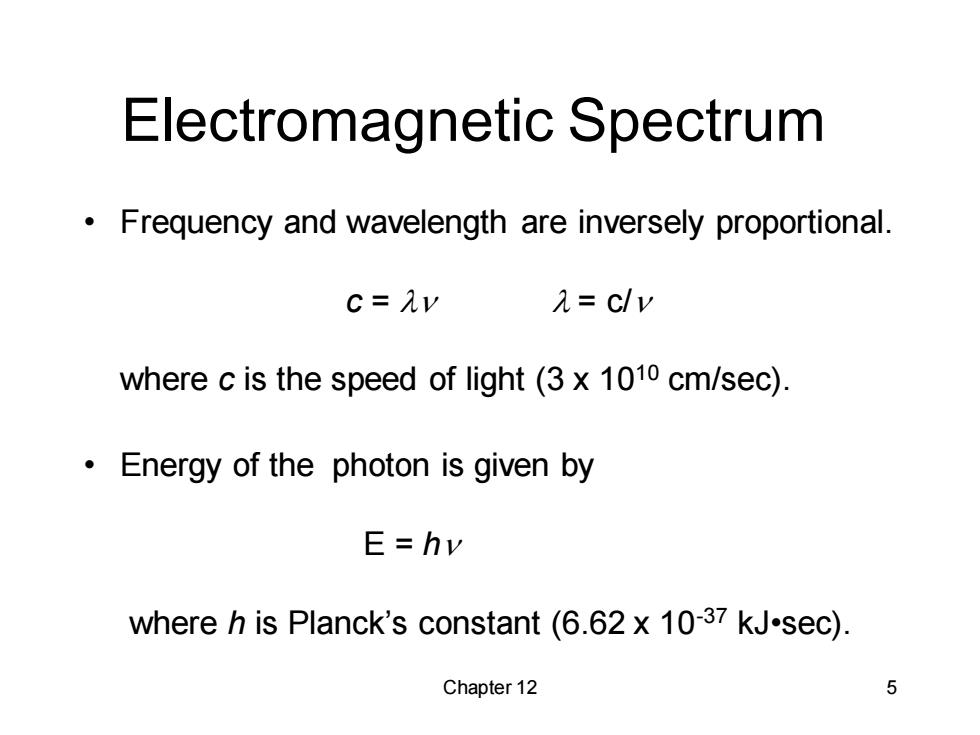
Electromagnetic Spectrum Frequency and wavelength are inversely proportional. c=Av 1=c/v where c is the speed of light (3 x 1010 cm/sec). Energy of the photon is given by E=hv where h is Planck's constant (6.62 x 10-37 kJ.sec). Chapter 12 5
Chapter 12 5 Electromagnetic Spectrum • Frequency and wavelength are inversely proportional. c = ln l = c/n where c is the speed of light (3 x 1010 cm/sec). • Energy of the photon is given by E = hn where h is Planck’s constant (6.62 x 10-37 kJ•sec)