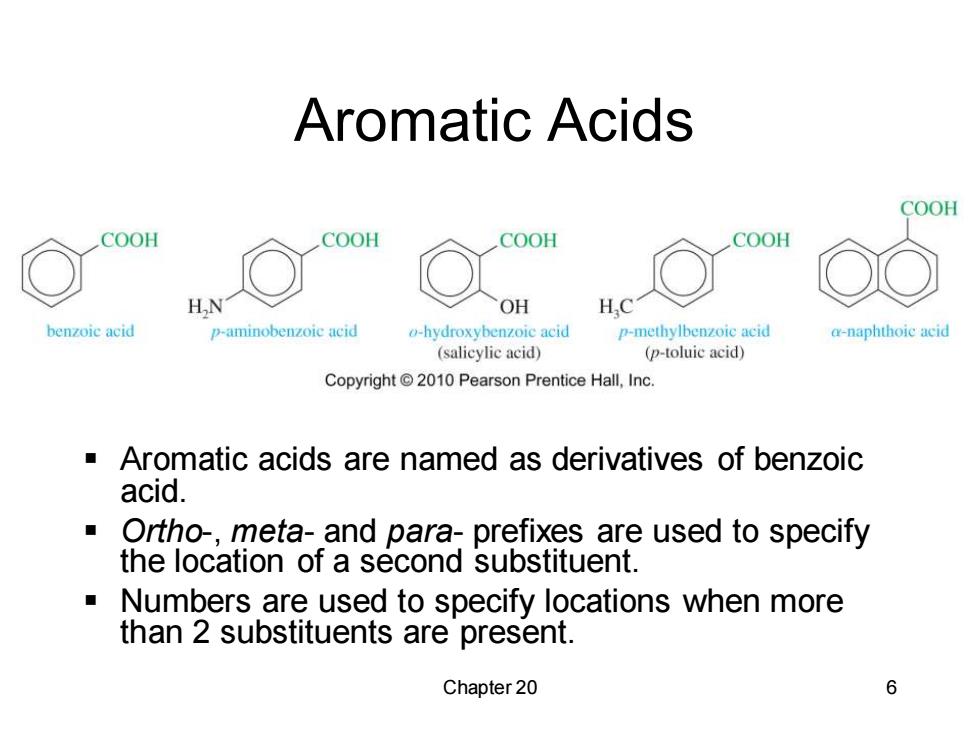
Aromatic Acids COOH COOH COOH COOH COOH HN OH H.C benzoic acid p-aminobenzoic acid o-hydroxybenzoic acid p-methylbenzoie aeid a-naphthoie acid (salicylic acid) (p-toluic acid) Copyright 2010 Pearson Prentice Hall,Inc. Aromatic acids are named as derivatives of benzoic acid. ■ Ortho-,meta-and para-prefixes are used to specify the location of a second substituent. Numbers are used to specify locations when more than 2 substituents are present. Chapter 20 6
Chapter 20 6 Aromatic Acids ▪ Aromatic acids are named as derivatives of benzoic acid. ▪ Ortho-, meta- and para- prefixes are used to specify the location of a second substituent. ▪ Numbers are used to specify locations when more than 2 substituents are present

Dicarboxylic Acids Aliphatic diacids are usually called by their common names. For IUPAC name,number the chain from the end closest to a substituent. Br HOOCCH2CHCH2CH2COOH 3-bromohexanedioic acid B-bromoadipic acid Chapter 20 7
Chapter 20 7 Dicarboxylic Acids ▪ Aliphatic diacids are usually called by their common names. ▪ For IUPAC name, number the chain from the end closest to a substituent. 3-bromohexanedioic acid -bromoadipic acid HOOCCH2CHCH2CH2COOH Br
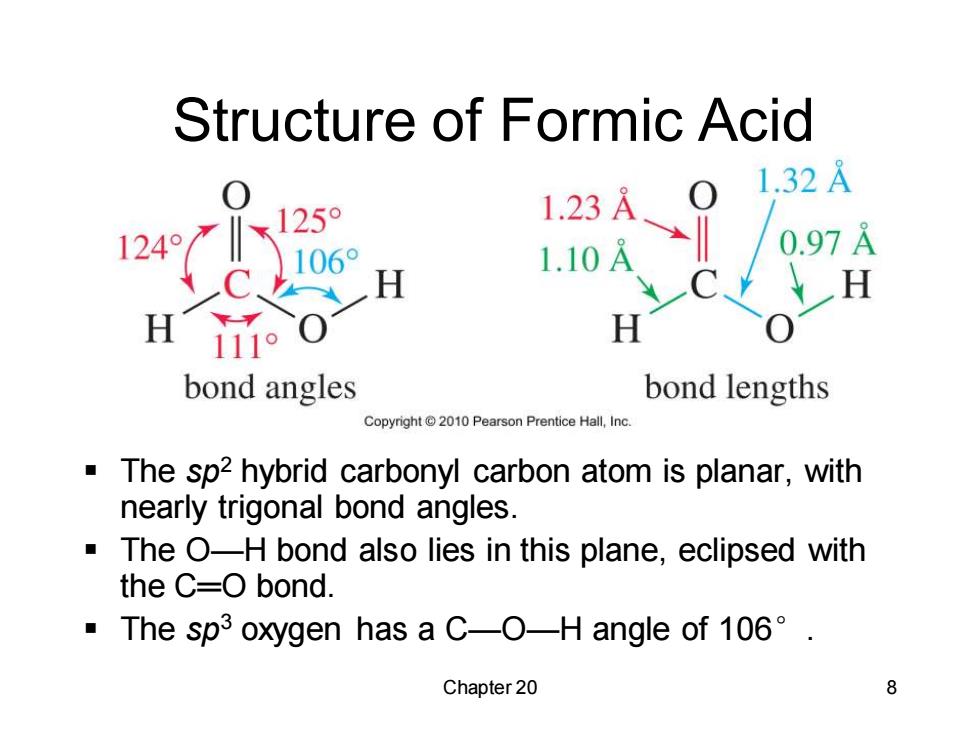
Structure of Formic Acid 1.32A 25° 1.23A 124° 1.10A 0.97A H H H 111o H bond angles bond lengths Copyright 2010 Pearson Prentice Hall,Inc The sp2 hybrid carbonyl carbon atom is planar,with nearly trigonal bond angles. The O-H bond also lies in this plane,eclipsed with the C=O bond. ·The spi3 oxygen has a C--O-H angle of106°. Chapter 20 8
Chapter 20 8 Structure of Formic Acid ▪ The sp2 hybrid carbonyl carbon atom is planar, with nearly trigonal bond angles. ▪ The O—H bond also lies in this plane, eclipsed with the C═O bond. ▪ The sp3 oxygen has a C—O—H angle of 106°
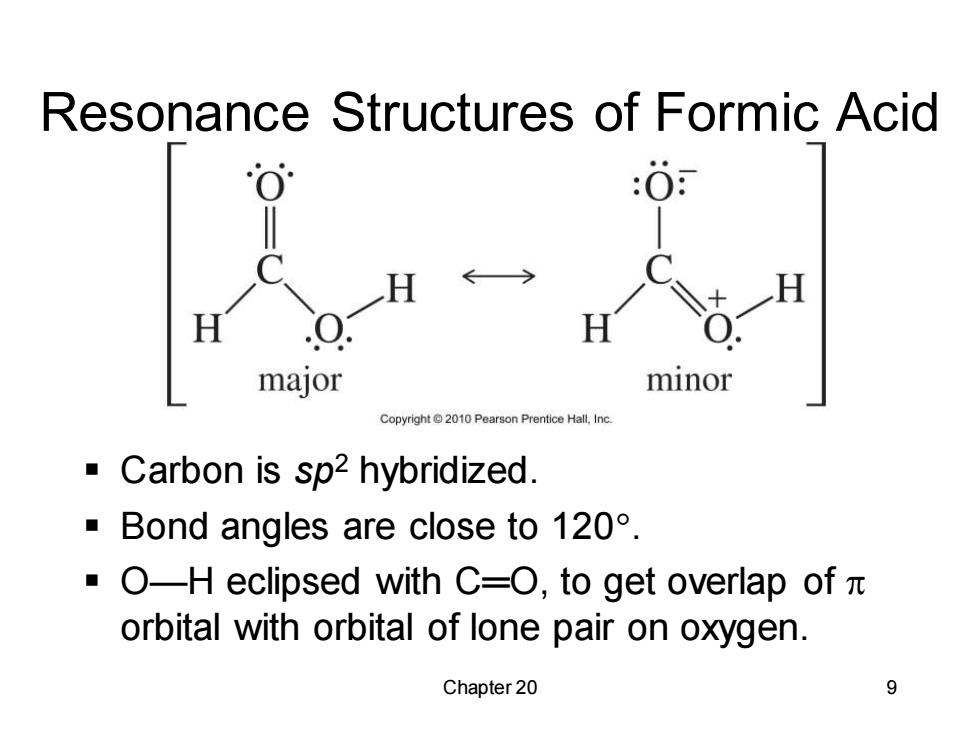
Resonance Structures of Formic Acid :0 H H major minor Copyright2010 Pearson Prentice Hall,Inc Carbon is sp2 hybridized. Bond angles are close to 120. ·O-H eclipsed with C=O,to get overlap ofπ orbital with orbital of lone pair on oxygen. Chapter 20 9
Chapter 20 9 Resonance Structures of Formic Acid ▪ Carbon is sp2 hybridized. ▪ Bond angles are close to 120. ▪ O—H eclipsed with C═O, to get overlap of orbital with orbital of lone pair on oxygen
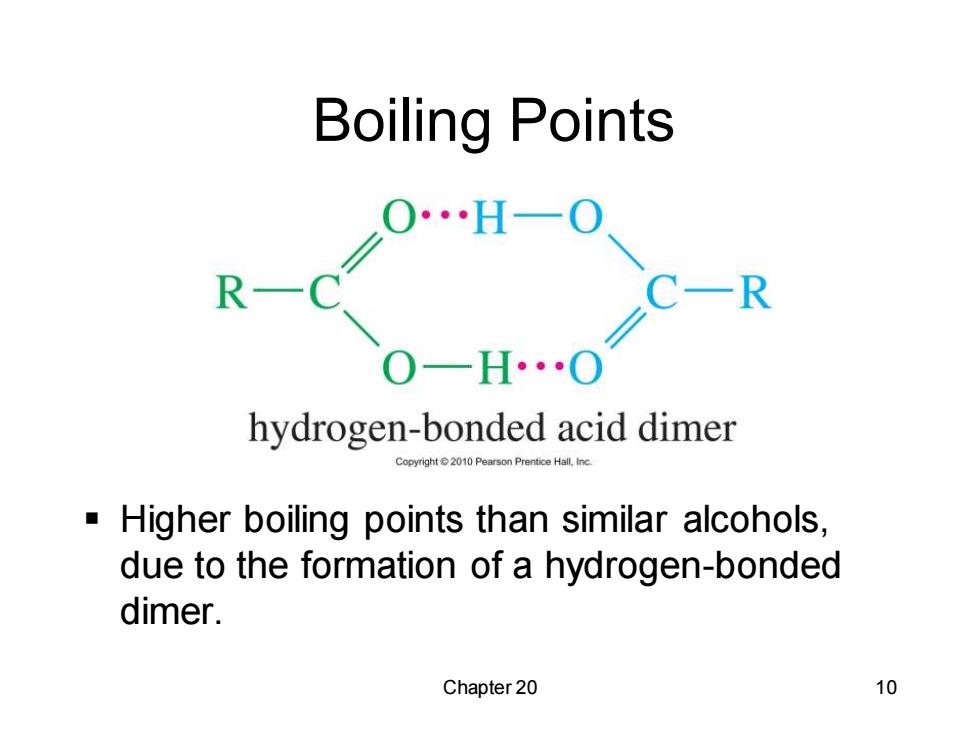
Boiling Points 20H-0 R C-R O-H..O hydrogen-bonded acid dimer Prentice Hall.Inc Higher boiling points than similar alcohols, due to the formation of a hydrogen-bonded dimer. Chapter 20 10
Chapter 20 10 Boiling Points ▪ Higher boiling points than similar alcohols, due to the formation of a hydrogen-bonded dimer