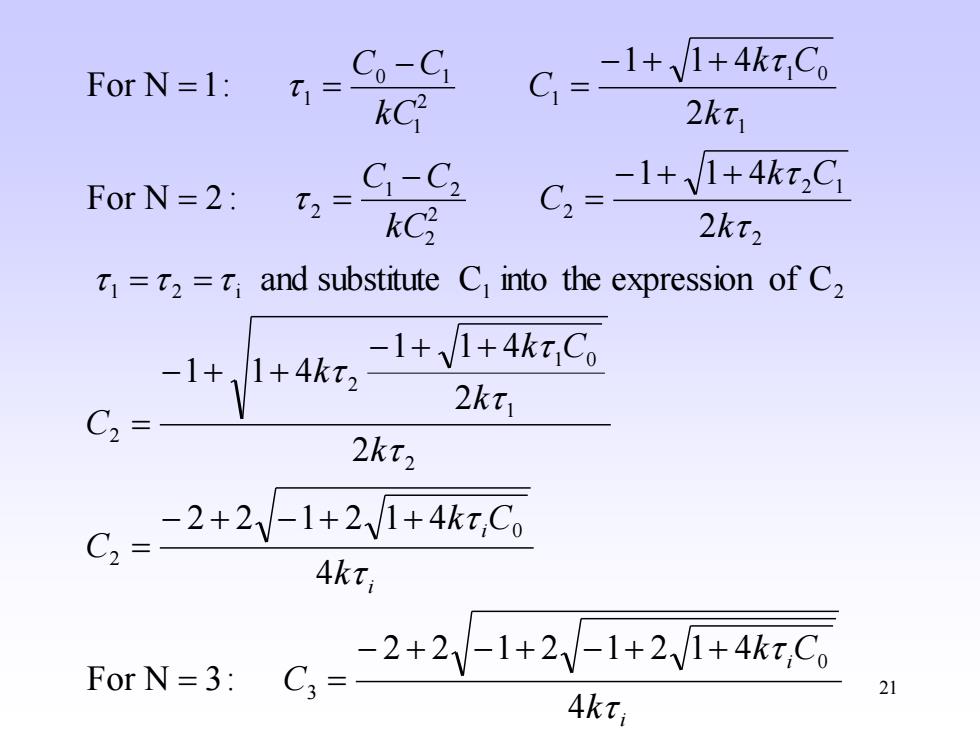
Co-Ci -1+1+4ktCo For N=1: t1= kC2 2kt For N=2: C1-C2 -1+V1+4kx2C kC2 2kt2 =2=;and substitute C into the expression of C2 -1+,1+4kt2 -1+1+4kt Co 2kt C2= 2kt2 C=2+2y-1+21+4x.G Akti For N=3: C=2+2y-1+21+24C 4kt; 21
21 i i i i k k C C k k C C k k k C k C k k C C k C C C k k C C k C C C 4 2 2 1 2 1 2 1 4 For N 3: 4 2 2 1 2 1 4 2 2 1 1 4 1 1 4 and substitute C into the expression of C 2 1 1 4 For N 2 : 2 1 1 4 For N 1: 0 3 0 2 2 1 1 0 2 2 1 2 i 1 2 2 2 1 2 2 2 1 2 2 1 1 0 2 1 1 0 1 1 − + − + − + + = = − + − + + = − + + − + + = = = − + + = − = = − + + = − = =
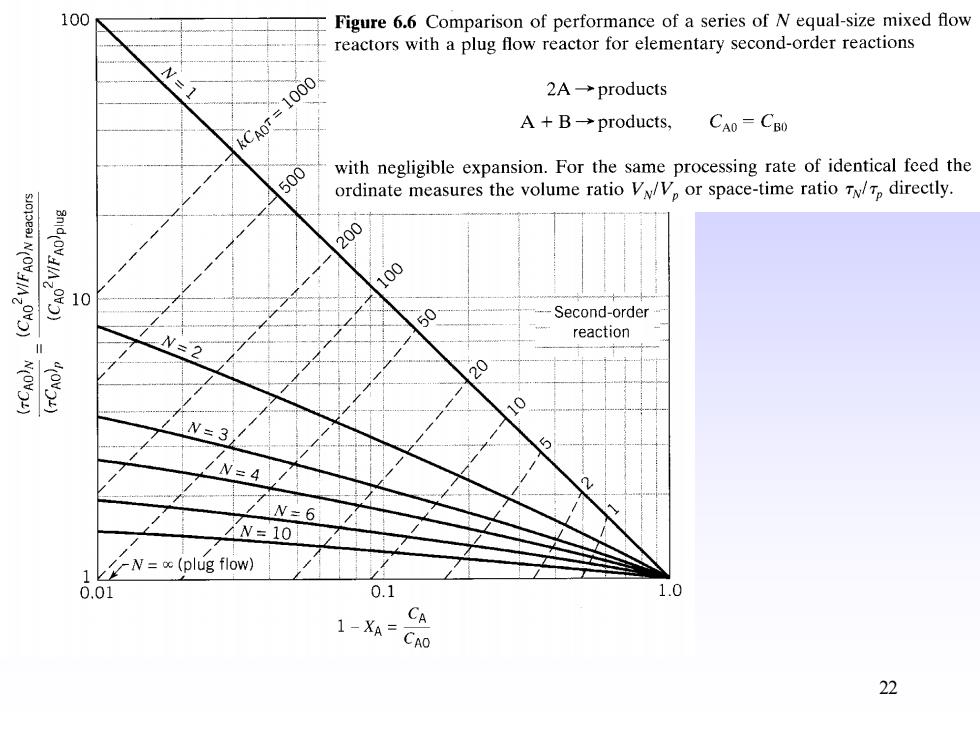
100 Figure 6.6 Comparison of performance of a series of N equal-size mixed flow reactors with a plug flow reactor for elementary second-order reactions kCA0r=1000 2A→products A+B→products,. CAO=CBD 500 with negligible expansion.For the same processing rate of identical feed the ordinate measures the volume ratio Va/Vp or space-time ratio Tp directly. N(OV) 200 10 50 Second-order reaction W=2 N(OV) 20 N=3 /N=4 N=6 N=10 N=o(plug flow) 0.01 0.1 1.0 1-XA= CA CAO 22
22
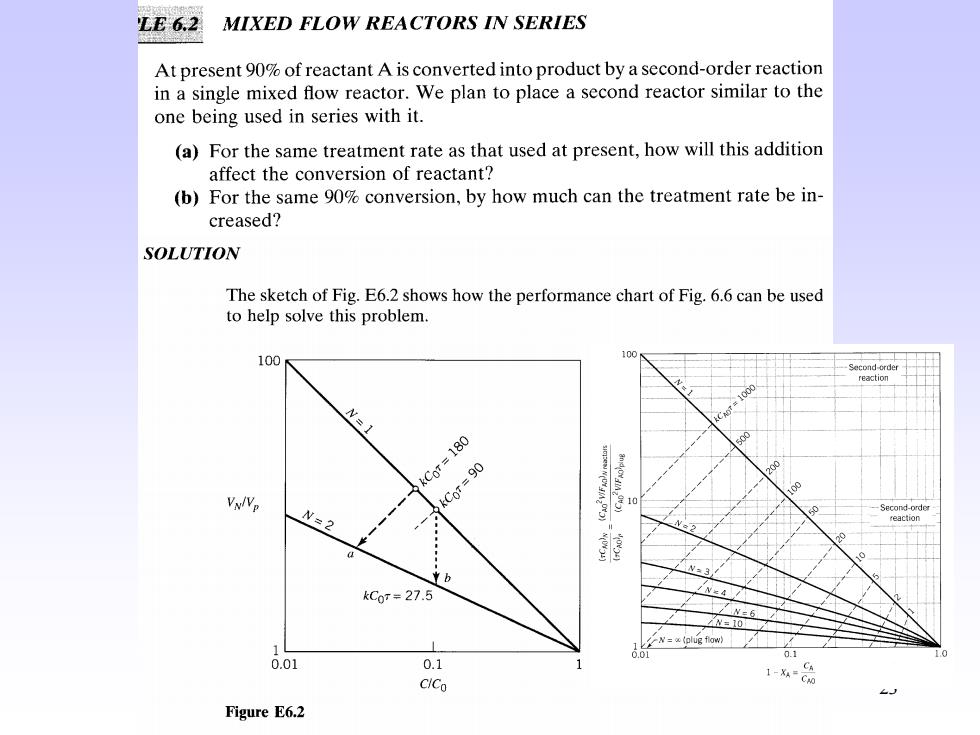
LE6.2 MIXED FLOW REACTORS IN SERIES At present 90%of reactant A is converted into product by a second-order reaction in a single mixed flow reactor.We plan to place a second reactor similar to the one being used in series with it. (a)For the same treatment rate as that used at present,how will this addition affect the conversion of reactant? (b)For the same 90%conversion,by how much can the treatment rate be in- creased? SOLUTION The sketch of Fig.E6.2 shows how the performance chart of Fig.6.6 can be used to help solve this problem. 100 100 N=1 kC0r=180 Vx/Vp kCoT=90 N=2 Second-order N=3 kC0r=27.5 N=x和lugfw 0.01 01 0.1 1 CiCo Figure E6.2
23
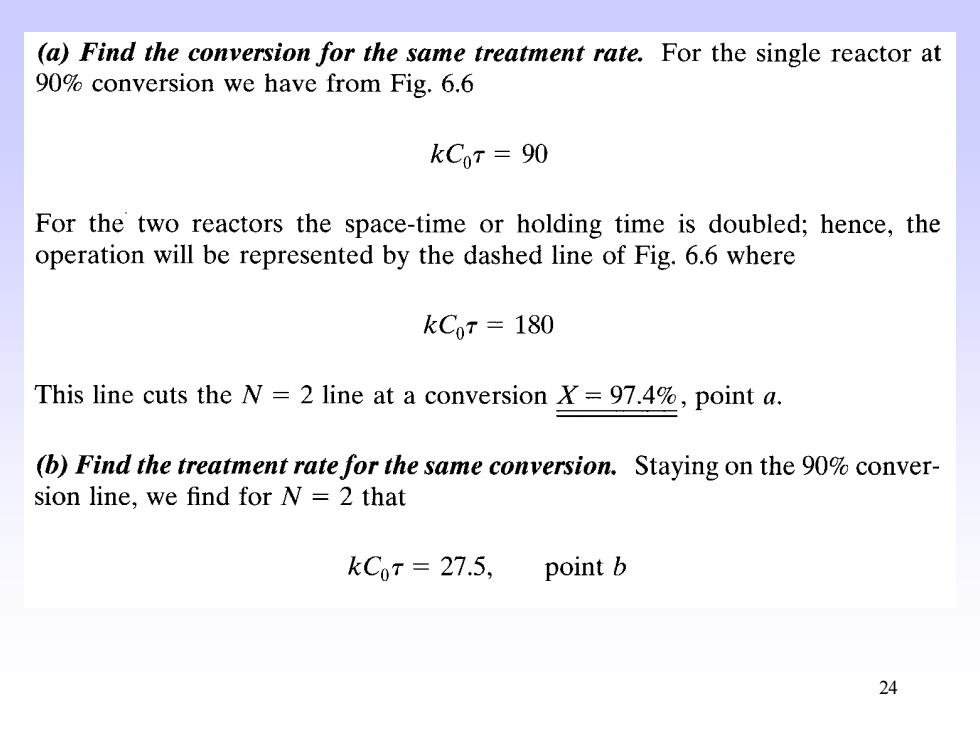
(a)Find the conversion for the same treatment rate.For the single reactor at 90%conversion we have from Fig.6.6 kCoT=90 For the two reactors the space-time or holding time is doubled;hence,the operation will be represented by the dashed line of Fig.6.6 where kC0r=180 This line cuts the N=2 line at a conversion X=97.4%,point a. (b)Find the treatment rate for the same conversion.Staying on the 90%conver- sion line,we find for N=2 that kCoT 27.5,point b 24
24

100 To double reactor size Divide reactor to two N=1 To lower conversion by 180 increasing treatment rate kCoT 9 TN=2 27.5 VNlVp TN-1 180 N=2 VN=2= 180 6.6 VN=I 27.5 Yb kC0r=27.5 1 0.01 0.1 1 CICo
25 To double reactor size Divide reactor to two To lower conversion by increasing treatment rate 6 6 27 5 180 v v 180 27 5 N 1 N 2 N 1 N 2 . . . = = = = = = =