
LOGO Reporting Results and Reliability of Analyses introduce Reporting Results 3 Reliability.of Analyses
LOGO Reporting Results and Reliability of Analyses 1 2 Reporting Results 3 Reliability of Analyses introduce
LOGO Reporting Results and Reliability of Analyses The basic purpose of an analytical assay is to determine the mass (weight)of a component in a sample.The numerical result of the assay is expressed as a weight percentage or in other units that are equivalent to the mass/mass ratio.The mass (weight)of a component in a food sample is calculated from a determination of a parameter whose magnitude is a function of the mass of the specific component in the sample. Some properties are basically mass dependent.Absorption of light or other forms of radiant energy is a function of the number of molecules,atoms,or ions in the absorbing species.Although certain properties,such as specific gravity and refractive index,are not mass dependent,they can be used indirectly for mass determination. Thus,one can determine the concentration of ethanol in aqueous solutions by a density determination.Refractive index is used routinely to determine soluble solids(mainly sugars)in syrups and jams.Some mass-dependent properties may be characteristic of several or even of a single component and may be used for selective and specific assays.Examples are light absorption, polarization,or radioactivity.Some properties have both a
LOGO The basic purpose of an analytical assay is to determine the mass (weight) of a component in a sample. The numerical result of the assay is expressed as a weight percentage or in other units that are equivalent to the mass/mass ratio. The mass (weight) of a component in a food sample is calculated from a determination of a parameter whose magnitude is a function of the mass of the specific component in the sample. Some properties are basically mass dependent. Absorption of light or other forms of radiant energy is a function of the number of molecules, atoms, or ions in the absorbing species. Although certain properties, such as specific gravity and refractive index, are not mass dependent, they can be used indirectly for mass determination. Thus, one can determine the concentration of ethanol in aqueous solutions by a density determination. Refractive index is used routinely to determine soluble solids (mainly sugars) in syrups and jams. Some mass-dependent properties may be characteristic of several or even of a single component and may be used for selective and specific assays. Examples are light absorption, polarization, or radioactivity. Some properties have both a Reporting Results and Reliability of Analyses

LOGO magnitude and a specificity parameter(nuclear magnetic resonance and infrared spectroscopy).Such properties are of great analytical value because they provide selective determinations of a relatively large number of substances. In this chapter,we describe conventional ways of expressing analytical results and discuss the significance of specificity,accuracy. precision,and sensitivity in assessing the reliability of analyses. In recent years the metric SI system of units has gained worldwide acceptance.It has been recommended or required by International Union of Pure and Applied Chemistry(IUPAC),and the International Union of Pure and Applied Physics(TUPAP),as well as by an increasing number of scientific and professional organizations in the United States and by the industry and the trade.The SI system contains seven base units,two supplementary units,15 derived units having special names,and 14 prefixes for multiple and submultiple units.All physical properties can be quantified by 38 names
LOGO magnitude and a specificity parameter (nuclear magnetic resonance and infrared spectroscopy). Such properties are of great analytical value because they provide selective determinations of a relatively large number of substances. In this chapter, we describe conventional ways of expressing analytical results and discuss the significance of specificity, accuracy, precision, and sensitivity in assessing the reliability of analyses. In recent years the metric SI system of units has gained worldwide acceptance. It has been recommended or required by International Union of Pure and Applied Chemistry (IUPAC), and the International Union of Pure and Applied Physics (IUPAP), as well as by an increasing number of scientific and professional organizations in the United States and by the industry and the trade. The SI system contains seven base units, two supplementary units, 15 derived units having special names, and 14 prefixes for multiple and submultiple units. All physical properties can be quantified by 38 names
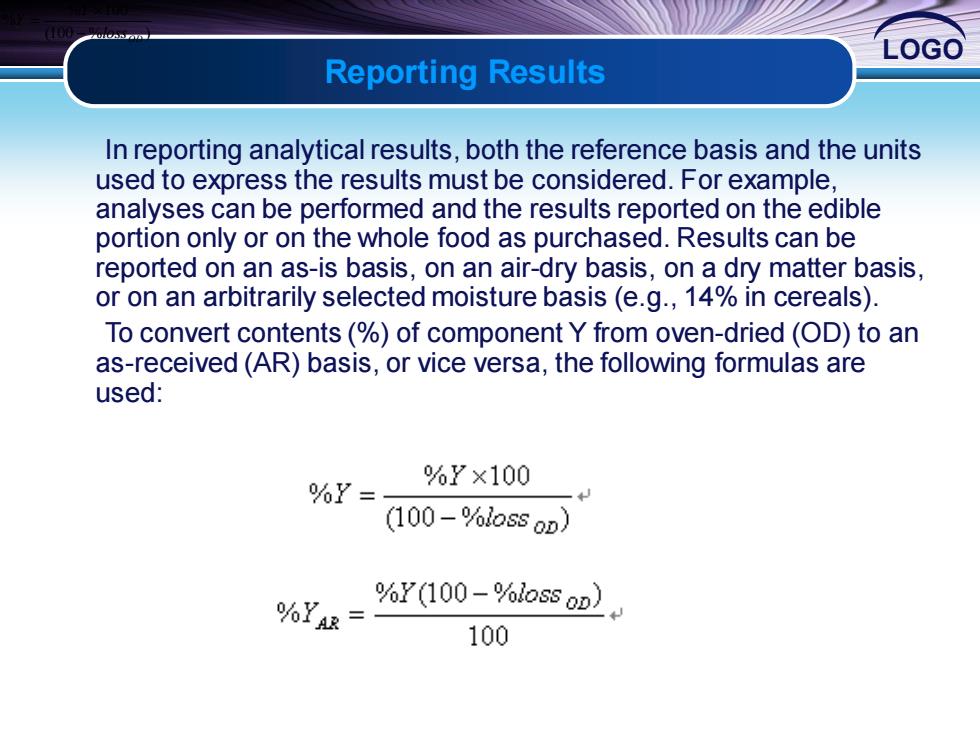
LOGO Reporting Results In reporting analytical results,both the reference basis and the units used to express the results must be considered.For example, analyses can be performed and the results reported on the edible portion only or on the whole food as purchased.Results can be reported on an as-is basis,on an air-dry basis,on a dry matter basis, or on an arbitrarily selected moisture basis (e.g.,14%in cereals). To convert contents(%)of component Y from oven-dried (OD)to an as-received(AR)basis,or vice versa,the following formulas are used: %Y= %Y×100 (100-%loss oD) %YAR= %Y(100-%loss oD) 100
LOGO In reporting analytical results, both the reference basis and the units used to express the results must be considered. For example, analyses can be performed and the results reported on the edible portion only or on the whole food as purchased. Results can be reported on an as-is basis, on an air-dry basis, on a dry matter basis, or on an arbitrarily selected moisture basis (e.g., 14% in cereals). To convert contents (%) of component Y from oven-dried (OD) to an as-received (AR) basis, or vice versa, the following formulas are used: Reporting Results (100 % ) % 100 % OD loss Y Y − =
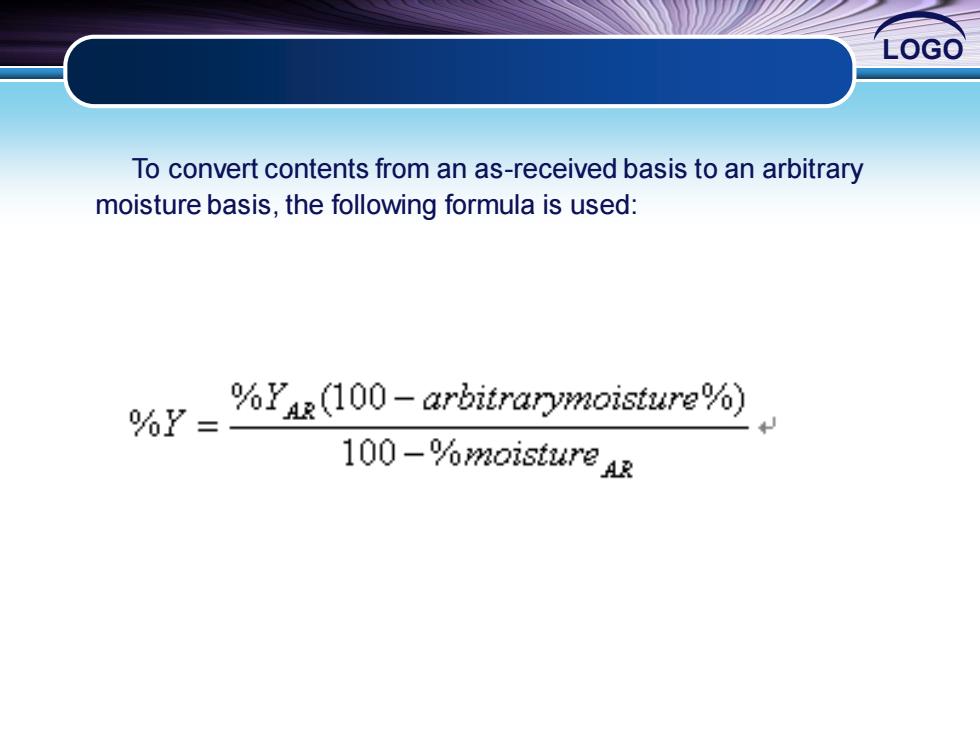
LOGO To convert contents from an as-received basis to an arbitrary moisture basis,the following formula is used: %Y= %YAR(100-arbitrarymoisture%) 100-%moisture Ar
LOGO To convert contents from an as-received basis to an arbitrary moisture basis, the following formula is used: