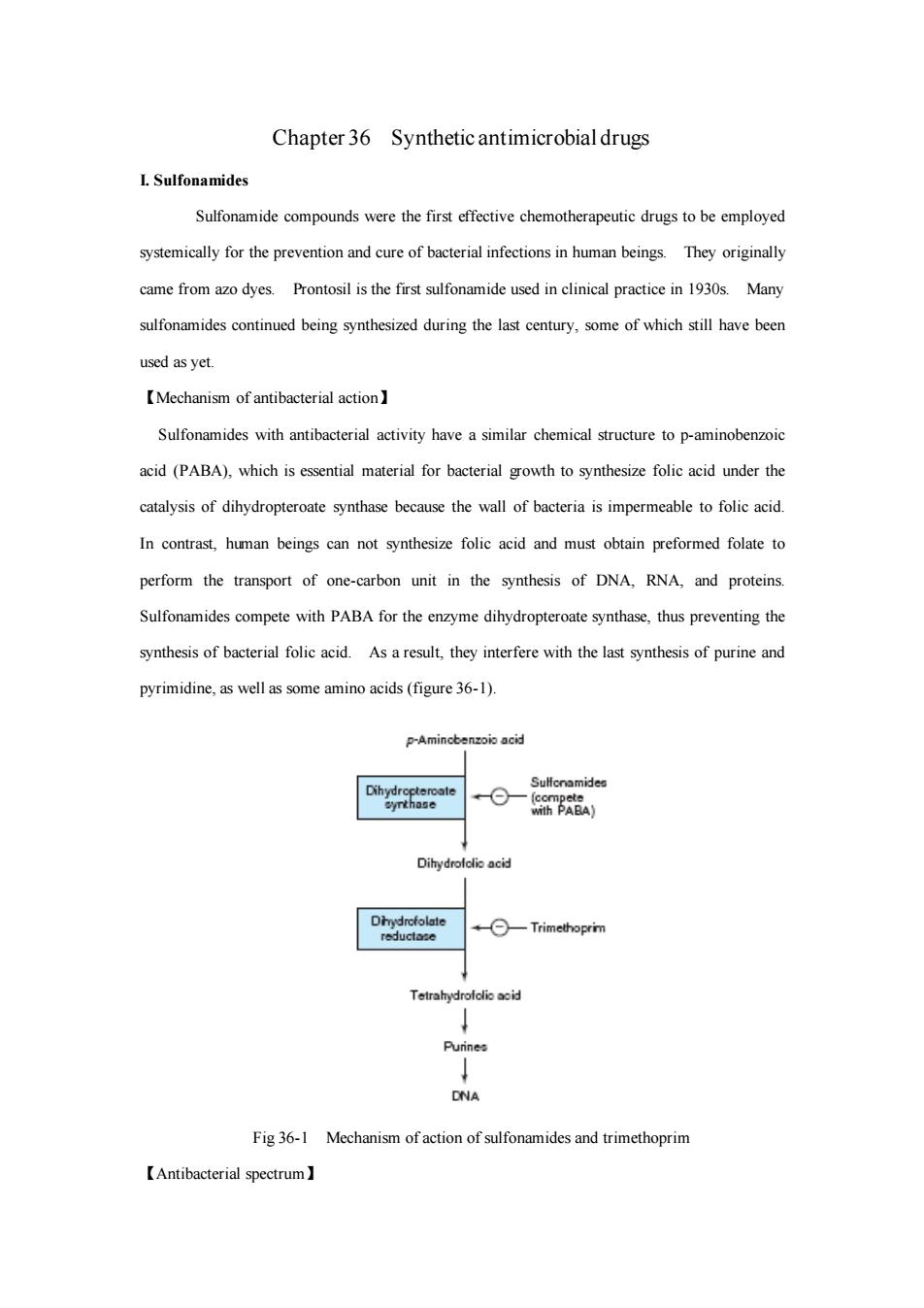
Chapter 36 Synthetic antimicrobial drugs I.Sulfonamides Sulfonamide compounds were the first effective chemotherapeutic drugs to be employed systemically for the prevention and cure of bacterial infections in human beings.They originally came from azo dyes.Prontosil is the first sulfonamide used in clinical practice in 1930s Many sulfonamides continued being synthesized during the last century,some of which still have been used as yet 【Mechanism of antibacterial action】 Sulfonamides with antibacterial activity have a similar chemical structure to p-aminobenzoic acid (PABA).which is essential material for bacterial growth to synthesize folic acid under the catalysis of dihydropteroate synthase because the wall of bacteria is impermeable to folic acid In contrast,human beings can not synthesize folic acid and must obtain preformed folate to perform the transport of one-carbon unit in the synthesis of DNA.RNA.and proteins. Sulfonamides compete with PABA for the enzyme dihydropteroate synthase,thus preventing the synthesis of bacterial folic acid.As a result,they interfere with the last synthesis of purine and pyrimidine,as well as some amino acids(figure 36-1). P-Ar Fig36-1 Mechanism of action of sulfonamides and trimethoprim 【Antibacterial spectrum】
Chapter 36 Synthetic antimicrobial drugs I. Sulfonamides Sulfonamide compounds were the first effective chemotherapeutic drugs to be employed systemically for the prevention and cure of bacterial infections in human beings. They originally came from azo dyes. Prontosil is the first sulfonamide used in clinical practice in 1930s. Many sulfonamides continued being synthesized during the last century, some of which still have been used as yet. 【Mechanism of antibacterial action】 Sulfonamides with antibacterial activity have a similar chemical structure to p-aminobenzoic acid (PABA), which is essential material for bacterial growth to synthesize folic acid under the catalysis of dihydropteroate synthase because the wall of bacteria is impermeable to folic acid. In contrast, human beings can not synthesize folic acid and must obtain preformed folate to perform the transport of one-carbon unit in the synthesis of DNA, RNA, and proteins. Sulfonamides compete with PABA for the enzyme dihydropteroate synthase, thus preventing the synthesis of bacterial folic acid. As a result, they interfere with the last synthesis of purine and pyrimidine, as well as some amino acids (figure 36-1). Fig 36-1 Mechanism of action of sulfonamides and trimethoprim 【Antibacterial spectrum】

Sulfonamides are bacteriostatic and inhibit both gram-positive and gram-negative bacteria, nocardia,Chlamydia trachomatis,some protozoa,and some enteric bacteria,such as E.coli. klebsiella,salmonella,shigella,and enterobacter.However,rickettsiae are not inhibited by sulfonamides but are actually stimulated in their growth. 【Resistance】 Bacteriaal resistance to sulfonamides can arise from plasmid transfers random mutations. The resistance in general,irreversible and may be caused by any offollowing three possibilities:1) the affinity of dihydropteroate synthase for sulfonamides decreases,and thus they become less effective competitors than PABA for this enzyme:2)Permeability to sulfonamides may be decreased insome,3)bacteria increase the production of PABA 【Pharmacokinetics】 Sulfonamides can be divided into three groups:1)oral,absorbable,2)oral,non-absorbable,3) topical.The oral,absorbable sulfonamides can be further divided into short-medium-,and ong-acting groupson th their half-lives.Only medium-acting groups are stll widely used for some bacterial infections.Sulfonamides are distributed in tissues and body fluids.They are metabolized by acetylation or glucuronidation in the liver and then excreted via urine. 【Clinicaluse】 1.Oral absorbable group Sulfisoxazole (6h)and sulfamethoxazole (h)are used to treat urinary tract infections.Sulfadiazine(t 13h)achieves high concentrations in CSF. and can be used with penicillin G for Neiseria meningitis or with pyrimethamine for toxoplasmosis.Sulfadoxime (8d)is available in combination with pyrimethamine as second-line drug for malaria 2.Oral non-absorbable group Sulfasalazine [sAlfo'salazin]is widely used in ulcerative coliisentriisand other inflammatory boweldisase. 3.Topical group Sodium sulfacetylamide] onment is effective treament for bacterialoivis and as adjunctive therapy for trachoma Silver sulfadiazine is available for prevention of infection of burn wounds. 【Adverse reactions】 Sulfonamides have a variety of side reactions due partly to allergy and partly to toxicity of the active drugs and their metabolites.Up to5of patients exhibit untoward reactions
Sulfonamides are bacteriostatic and inhibit both gram-positive and gram-negative bacteria, nocardia, Chlamydia trachomatis, some protozoa, and some enteric bacteria, such as E.coli, klebsiella, salmonella, shigella, and enterobacter. However, rickettsiae are not inhibited by sulfonamides but are actually stimulated in their growth. 【Resistance】 Bacteriaal resistance to sulfonamides can arise from plasmid transfers or random mutations. The resistance in general, irreversible and may be caused by any of following three possibilities: 1) the affinity of dihydropteroate synthase for sulfonamides decreases, and thus they become less effective competitors than PABA for this enzyme; 2) Permeability to sulfonamides may be decreased in some resistant strains; 3) bacteria increase the production of PABA. 【Pharmacokinetics】 Sulfonamides can be divided into three groups: 1) oral, absorbable; 2) oral, non-absorbable; 3) topical. The oral, absorbable sulfonamides can be further divided into short- medium-, and long-acting groups on the their half-lives. Only medium-acting groups are still widely used for some bacterial infections. Sulfonamides are distributed in tissues and body fluids. They are metabolized by acetylation or glucuronidation in the liver and then excreted via urine. 【Clinical use】 1. Oral absorbable group Sulfisoxazole (t1/2 6h) and sulfamethoxazole (t1/2 11h) are used to treat urinary tract infections. Sulfadiazine (t1/2 13h) achieves high concentrations in CSF, and can be used with penicillin G for Neiseria meningitis or with pyrimethamine for toxoplasmosis. Sulfadoxine (t1/2 8d) is available in combination with pyrimethamine as second- line drug for malaria. 2. Oral non-absorbable group Sulfasalazine [slf’sælzin] is widely used in ulcerative colitis, enteritis, and other inflammatory bowel disease. 3. Topical group Sodium sulfacetylamide [sכdim slf’setilæmaid] ophthalmic solution or ointment is effective treatment for bacterial conjunctivitis and as adjunctive therapy for trachoma. Silver sulfadiazine is available for prevention of infection of burn wounds. 【Adverse reactions】 Sulfonamides have a variety of side reactions due partly to allergy and partly to toxicity of the active drugs and their metabolites. Up to 5 % of patients exhibit untoward reactions

including:1)allergic reactions and reactions with other sulfonamide derivatives. such as thiazides diuretics,sulfonaurea,diazoxide,furosemide,and bumetanide:2)crystalluria and hematuria,which may be decreased by adequate hydration and alkalinization of urine:3) hematopoietic disturbances.including hemolytic anemia in patients with G-6-PD deficiency. granulocytopenia,and thrombocytopenia I.Trimethoprim Trimethoprim is a potent inhibitor of bacterial dihydrofolate reductase,and prevents dihydrofolic acid to become tetrahydrofolic acid.It exhibits an antibacterial spectrum similar to the sulfonamides,but the antibacterial potency is 20 to50 time more than sulfonamide It is absorbed well after oral administration and has similar pharmacokinetic properties to sulfamethoxazole Therefore,it has been fommulated with sulfamethoxazole as co-rim to treat the patients with mild bacterial infections.Trimethoprim can produce the effects of folate deficiency,that may cause megaloblastic anemia leukopenia,and granulocytopenia IIL.Fluoquinolones Nalidixic acid is ancestor offluoqunoeswhich had been discarded in190sbecause of less effectivenes against bacteria and much more adverse reactions.However,since late1970 many fluoquinolones have been being developed in some pharmaceutic companies of the world. They quickly occupy the part of market of antimicrobial drugs. 【Mechanism of antibacterial action】 As well known that topoisomerase II is the enzyme that changes the configuration or topology of DNA by nicking pass-through,and re-sealing mechanism without changing DNA primary structure during the period of DNA replication.Topoisomerase IV plays action on separation of interlinked (catenated)daughter DNA molecules that are the product of DNA replication.DNA gyrase of bacteria is topoismerase II.Fluquinolones block bacterial DNA synthesis by inhibiting bacterial DNA gyrase and Topoisomerase IV.Thus,they interfere with the normal replication and transription of bacterial DNA at the conenraions of10gm and lead to cell death Eukaryotic cells do not contain DNA gyrase.But they contain a conceptually and mechanistically similar topoisomerase II that removes positive supercoils from
including: 1) allergic reactions and cross-allergic reactions with other sulfonamide derivatives, such as thiazides diuretics, sulfonaurea, diazoxide, furosemide, and bumetanide; 2) crystalluria and hematuria, which may be decreased by adequate hydration and alkalinization of urine; 3) hematopoietic disturbances, including hemolytic anemia in patients with G-6-PD deficiency, granulocytopenia, and thrombocytopenia. II. Trimethoprim Trimethoprim is a potent inhibitor of bacterial dihydrofolate reductase, and prevents dihydrofolic acid to become tetrahydrofolic acid. It exhibits an antibacterial spectrum similar to the sulfonamides, but the antibacterial potency is 20 to 50 time more than sulfonamide. It is absorbed well after oral administration and has similar pharmacokinetic properties to sulfamethoxazole. Therefore, it has been formulated with sulfamethoxazole as co-trimoxazole to treat the patients with mild bacterial infections. Trimethoprim can produce the effects of folate deficiency, that may cause megaloblastic anemia, leukopenia, and granulocytopenia. III. Fluoquinolones Nalidixic acid is ancestor of fluoquinolones, which had been discarded in 1960s because of less effectiveness against bacteria and much more adverse reactions. However, since late 1970s many fluoquinolones have been being developed in some pharmaceutic companies of the world. They quickly occupy the part of market of antimicrobial drugs. 【Mechanism of antibacterial action】 As well known that topoisomerase II is the enzyme that changes the configuration or topology of DNA by nicking, pass-through, and re-sealing mechanism without changing DNA primary structure during the period of DNA replication. Topoisomerase IV plays action on separation of interlinked (catenated) daughter DNA molecules that are the product of DNA replication. DNA gyrase of bacteria is topoisomerase II. Fluoquinolones block bacterial DNA synthesis by inhibiting bacterial DNA gyrase and Topoisomerase IV. Thus, they interfere with the normal replication and transcription of bacterial DNA at the concentrations of 0.1-10 g/ml and lead to cell death. Eukaryotic cells do not contain DNA gyrase. But they contain a conceptually and mechanistically similar topoisomerase II that removes positive supercoils from

eukaryotic DNA to prevent its tanging during replication.Fluoquinolones inhibit eukaryotic topoisomerase Ilony at the leves of 100-1000 Hg/ml). Fig 36-2 Model of the formation of negative DNA supercoils by DNA gyrase 【Antibacterial spectrum and use】 All fluoquinolones are bactericidal.Generally,they are effective against gram-negative bacteria such as enterobacteria,pseudomonas organisms,Haemophilus influenzae,Moraxella catarrhalis,Legionella.Chlamydia,and mycobacteria.They are effective in the treatment of gonorrhea but not syphilis Though active against some gram-positive bacteria,they should be avoided to treat the patients with pneumococcal or enterococcal infections.They have poor activity on anaerobes. 1.Ciprofloxacin Ciprofloxacin is the most potent of fluoquinolones and an good alternative to more toxic drugs,such as aminoglycosides It may has synergistical activity wth B-lactams against some bacterial infections and often effective in treatment of infections unresponsive to ampicillin,amoxicillin,and bacampicillin.Ciprofloxacin is often used in treament of rinary Itis highly in treatingacute enteric pathologens Ciprofloxacin also can be used for many infectious diseases due to gonorrhea.Enterobactor.E coli.Klebsiella pneumoniae.Haemophilus influerae.Legionella pneumophila.Pseudomonas aeruginosa.Proteus mirabilis.Serrana marcescens.and shigella But like other fluoquinoloesit has weak action on Streptococcus pneumoniae 2.Norfoxacin Norfloxacin has an antibacterial spectrum similar to that of ciprofloxacin and is used to treat the infectious diseases from gram-negative and gram-positive organisms. 3.Ofloxacin is primarily used in the treatment of prostatitis caused by E.co and of sxally transmitted diseases except syphilis.It may be used as alterative therapy in
eukaryotic DNA to prevent its tanging during replication. Fluoquinolones inhibit eukaryotic topoisomerase II only at the leves of 100-1000 g/ml). Fig 36-2 Model of the formation of negative DNA supercoils by DNA gyrase 【Antibacterial spectrum and clinical use】 All fluoquinolones are bactericidal. Generally, they are effective against gram-negative bacteria such as enterobacteria, pseudomonas organisms, Haemophilus influenzae, Moraxella catarrhalis, Legionella, Chlamydia, and mycobacteria. They are effective in the treatment of gonorrhea but not syphilis. Though active against some gram-positive bacteria, they should be avoided to treat the patients with pneumococcal or enterococcal infections. They have poor activity on anaerobes. 1. Ciprofloxacin Ciprofloxacin is the most potent of fluoquinolones and an good alternative to more toxic drugs,such as aminoglycosides. It may has synergistical activity wth -lactams against some bacterial infections and often effective in treatment of infections unresponsive to ampicillin, amoxicillin, and bacampicillin. Ciprofloxacin is often used in treatment of urinary infections. It is highly efficacious in treating acute diarrheal illnesses due to enteric pathologens. Ciprofloxacin also can be used for many infectious diseases due to gonorrhea, Enterobactor, E coli, Klebsiella pneumoniae, Haemophilus influenzae, Legionella pneumophila, Pseudomonas aeruginosa, Proteus mirabilis, Serratia marcescens, and shigella. But like other fluoquinolones, it has weak action on Streptococcus pneumoniae. 2. Norfloxacin Norfloxacin has an antibacterial spectrum similar to that of ciprofloxacin and is used to treat the infectious diseases from gram-negative and gram-positive organisms. 3. Ofloxacin Ofloxacin is primarily used in the treatment of prostatitis caused by E. coli and of sexually transmitted diseases except syphilis. It may be used as alternative therapy in

patients with gonorrhea.It has some benefit in the treatment of skin and lower respiratory tract infections. 4.Lomefloxacin and enoxacin Lomefloxacin and enoxacin are useful in the treatment of urethral tract infection and bronchitisdue to Haemoohilus influenzaeor Moraxella catarhalis. 5.Trovafloxacin(Trovafloxin)Trovafloxacin is anew fluoroquinolone,which has several properties that give it some clinical advantages.These propertics include a long half-life of about 10 hours,which permits on-a-day dosing.and a extended spetrum of antimicrobial activity including most anaerobes.However,trovafloxacin has aserious toxicity of liver injury. and use of the drug is restricted to infections that are life-threatening (for example,pneumonia, intra-abdominal infections,gynecologic and pelvic infections).The therapy with trovafloxacin should not for longer than 14 days.anf should only be used in hospital and long-term nursing care facilities 【Resistance】 When the were first introduced,there was optimism that resistance would not develop.Although no plasmid-mediated resistance has been reported,esistance of MRSA,pseudomonas coagulase-negative staphylococci and enterococci has unfortunately emerged due to chromosomal mutations.Cross-resistance exists among the quinolones.The mechanisms responsible for this resistance are:1)Modifications in the bacterial DNA gyrase. especially in amino acids at the N-terminus of the A subunit,have been associated with decreased affinity for fluoroquinolones.2)Accumulation of fluoroquinolones in bacteria decreases because of a decreased number of porin proteins in the outer membrane and disorder of an energy-dependent efflux system. 【Pharmacokinetics】 After oral administration,fluoroquinoloes are well absorbed (bioavailability of8%)and distributed widely in body fluids and tissues.Serun elinination half-lives rangs from3 hours to 18 hours (Table 36-1).Oral absorption is impaired by divalent cations.Most of fluoroquinolones are eliminated by kidneys
patients with gonorrhea. It has some benefit in the treatment of skin and lower respiratory tract infections. 4. Lomefloxacin and enoxacin Lomefloxacin and enoxacin are useful in the treatment of urethral tract infection and bronchitis due to Haemoohilus influenzae or Moraxella catarrhalis. 5. Trovafloxacin (Trovafloxin) Trovafloxacin is anew fluoroquinolone, which has several properties that give it some clinical advantages. These properties include a long half-life of about 10 hours, which permits once-a-day dosing, and a extended spetrum of antimicrobial activity including most anaerobes. However, trovafloxacin has a serious toxicity of liver injury, and use of the drug is restricted to infections that are life-threatening (for example, pneumonia, intra-abdominal infections, gynecologic and pelvic infections). The therapy with trovafloxacin should not continue for longer than 14 days, anf should only be used in hospital and long-term nursing care facilities. 【Resistance】 When the fluoroquinolones were first introduced, there was optimism that resistance would not develop. Although no plasmid-mediated resistance has been reported, esistance of MRSA, pseudomonas, coagulase-negative staphylococci and enterococci has unfortunately emerged due to chromosomal mutations. Cross-resistance exists among the quinolones. The mechanisms responsible for this resistance are: 1) Modifications in the bacterial DNA gyrase, especially in amino acids at the N-terminus of the A subunit, have been associated with decreased affinity for fluoroquinolones. 2) Accumulation of fluoroquinolones in bacteria decreases because of a decreased number of porin proteins in the outer membrane and disorder of an energy-dependent efflux system. 【Pharmacokinetics】 After oral administration, fluoroquinolones are well absorbed (bioavailability of 80-95%) and distributed widely in body fluids and tissues. Serun elinination half-lives rangs from 3 hours to 18 hours (Table 36-1). Oral absorption is impaired by divalent cations. Most of fluoroquinolones are eliminated by kidneys