
2化学电离源(chemical ionization CD) 结构与EI同 原理: 若以甲烷作为反应气,电离过程如下: CH e=CH++CH3*+CH2++CH++C+ CH4++CH4→CH++CH CHL3++CH4→C2H++2 CHL++XH→XH2+CH4 C2H,++XH→X++C2H6 特点:得准分子离子,得不到标准质谱
2 化学电离源(chemical ionization CI) CH4 + e CH4 + + CH3 + + CH2 + + CH + + C+ CH4 + + CH4 CH5 + + CH3 CH3 + + CH4 C2H5 + + H2 CH5 + + XH XH2 + CH4 C2H5 + + XH X + + C2H6 特点:得准分子离子,得不到标准质谱 结构与EI同 原理: 若以甲烷作为反应气,电离过程如下:
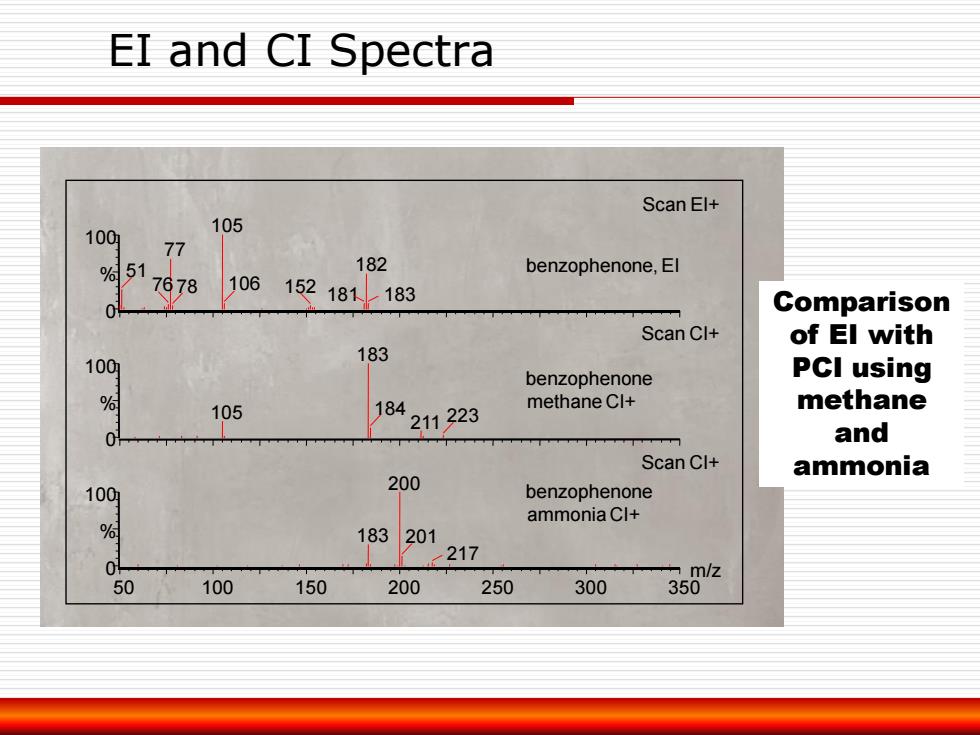
EI and CI Spectra Scan El+ 105 100 77 7978 % 51 182 benzophenone,El 106 152181↓183 Comparison Scan Cl+ of El with 183 100 benzophenone PCI using % 105 18 methane 211223 methane Cl+ and Scan Cl+ ammonia 1009 200 benzophenone ammonia Cl+ 183 201 0 217 m/z 50 100 150 200 250 300 350
EI and CI Spectra 50 100 150 200 250 300 350 0 m/z 100 % 0 100 % 0 100 % 200 183 201 217 Scan CI+ 183 105 184 211 223 Scan CI+ 105 77 51 76 78 182 106 181 152 183 Scan EI+ benzophenone, EI benzophenone methane CI+ benzophenone ammonia CI+ Comparison of EI with PCI using methane and ammonia
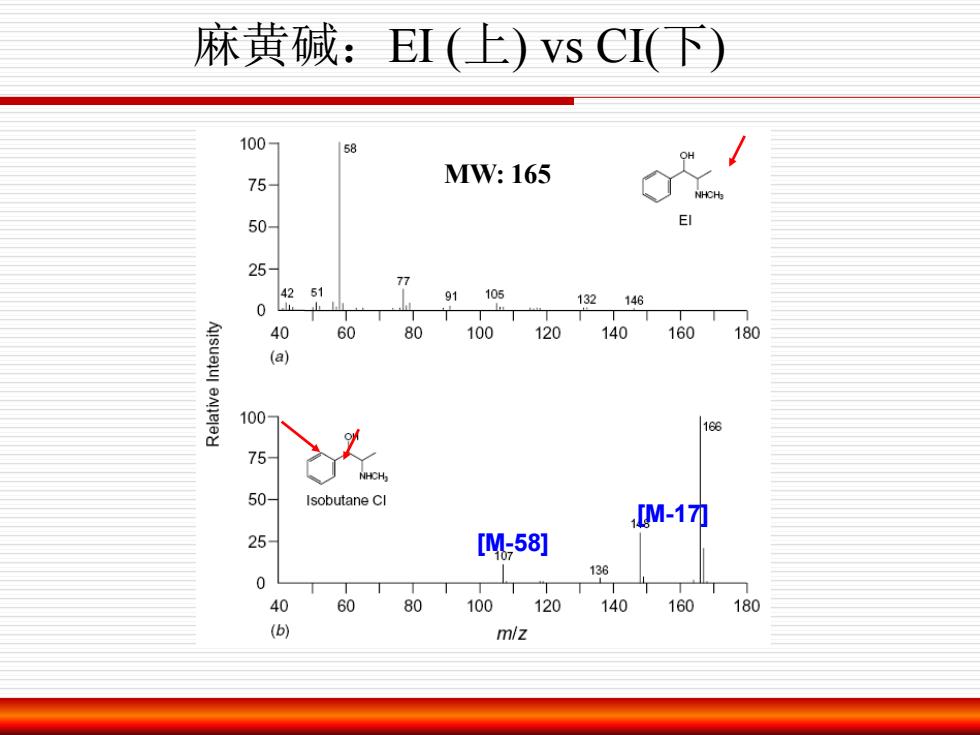
麻黄碱:EI(上)vsCI(下) 100 75- MW:165 50 25 77 4251 91105 132146 0 40 60 80 100120 140160 180 (a) 100 16 NHCH 50- Isobutane CI M-17力 25 [W58] 136 0 40 60 80 100 120 140 160 180 () m/z
MW: 165 [M-17] [M-58] 麻黄碱:EI (上) vs CI(下)

Butyl methacrylate (MW:142):EI vs CI 8000 69 6000 56 4000 2000 87 82100 40 60 80 100 120 140 160 180 m/z 4500 87 CI(methane) 3000 1500 69 143 6181100115127 60 80 100120140 160 143 1200 CI (isobutane 900 600 300 7387 971 60 80 100120140 160m280
Butyl methacrylate (MW:142): EI vs CI EI CI (methane) CI (isobutane )
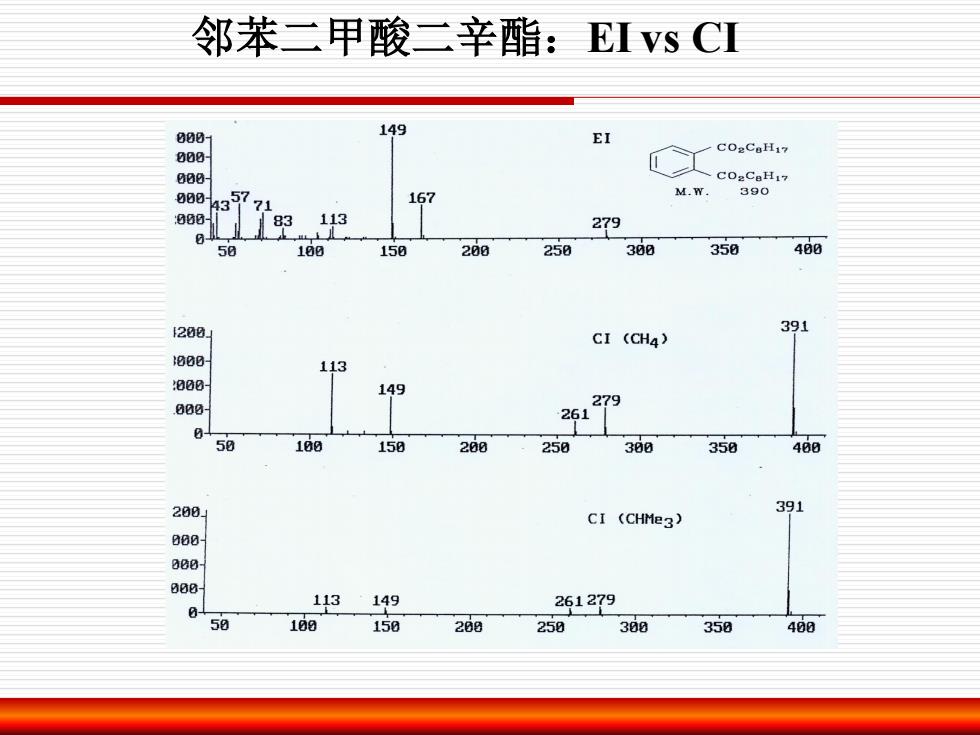
邻苯二甲酸二辛酯:EI vs CI 000 149 EI 000 COaCaH17 800 000 9357 167 M.W. 390 71 00 113 279 m 58 100 150 200 250 300 350 400 200 391 CI (CH4) 800 113 800 eee 9 26127 50 100 158 288 250 300 350 488 288 391 CI (CHMe3) 8 113 149 261279 50 16g 150 268 259 360 350 400
邻苯二甲酸二辛酯:EI vs CI