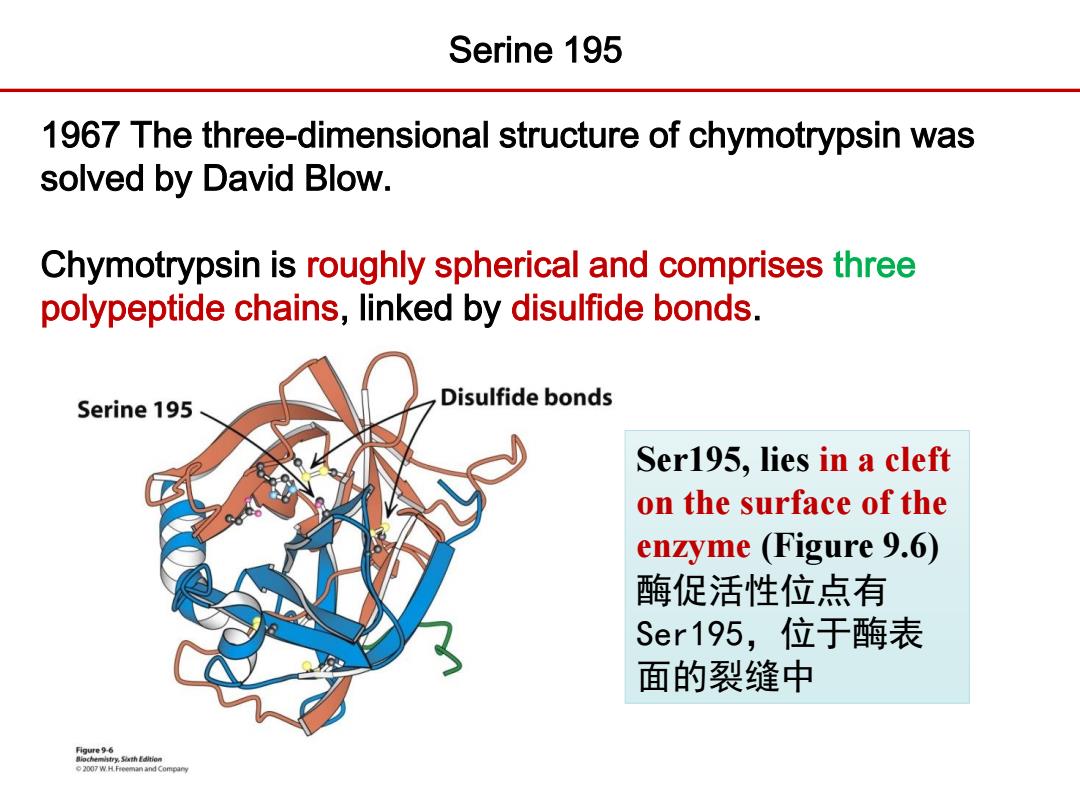
Serine 195 1967 The three-dimensional structure of chymotrypsin was solved by David Blow. Chymotrypsin is roughly spherical and comprises three polypeptide chains,linked by disulfide bonds. Serine 195 Disulfide bonds Ser195,lies in a cleft on the surface of the enzyme (Figure 9.6) 酶促活性位点有 Ser195,位于酶表 面的裂缝中 2007 W.H.Freeman and Company
1967 The three-dimensional structure of chymotrypsin was solved by David Blow. Chymotrypsin is roughly spherical and comprises three polypeptide chains, linked by disulfide bonds. Serine 195 Ser195, lies in a cleft on the surface of the enzyme (Figure 9.6) 酶促活性位点有 Ser195,位于酶表 面的裂缝中
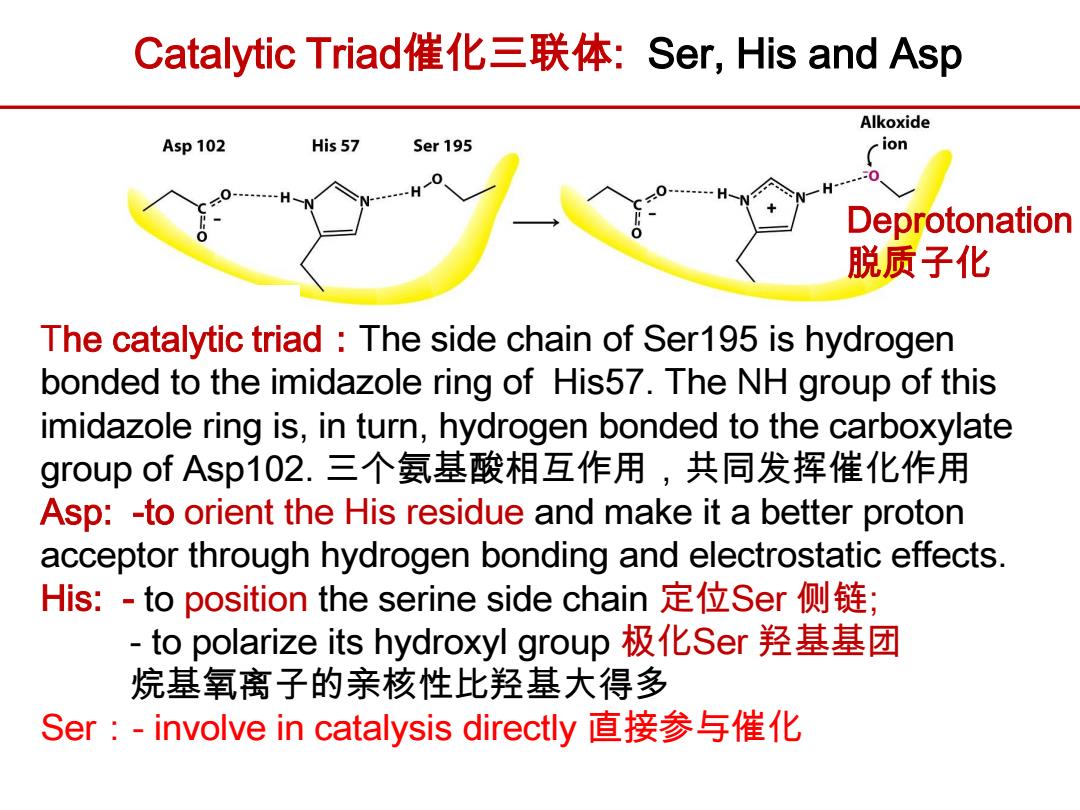
Catalytic Triad催化三联体:Ser,His and Asp Alkoxide Asp 102 His57 Ser 195 ion 0 Deprotonation 脱质子化 The catalytic triad The side chain of Ser195 is hydrogen bonded to the imidazole ring of His57.The NH group of this imidazole ring is,in turn,hydrogen bonded to the carboxylate group of Asp102.三个氨基酸相互作用,共同发挥催化作用 Asp:-to orient the His residue and make it a better proton acceptor through hydrogen bonding and electrostatic effects. His:-to position the serine side chain定位Ser侧链; -to polarize its hydroxyl group极化Ser羟基基团 烷基氧离子的亲核性比羟基大得多 Ser:-involve in catalysis directly直接参与催化
The catalytic triad:The side chain of Ser195 is hydrogen bonded to the imidazole ring of His57. The NH group of this imidazole ring is, in turn, hydrogen bonded to the carboxylate group of Asp102. 三个氨基酸相互作用,共同发挥催化作用 Asp: -to orient the His residue and make it a better proton acceptor through hydrogen bonding and electrostatic effects. His: - to position the serine side chain 定位Ser 侧链; - to polarize its hydroxyl group 极化Ser 羟基基团 烷基氧离子的亲核性比羟基大得多 Ser:- involve in catalysis directly 直接参与催化 Catalytic Triad催化三联体: Ser, His and Asp Deprotonation 脱质子化
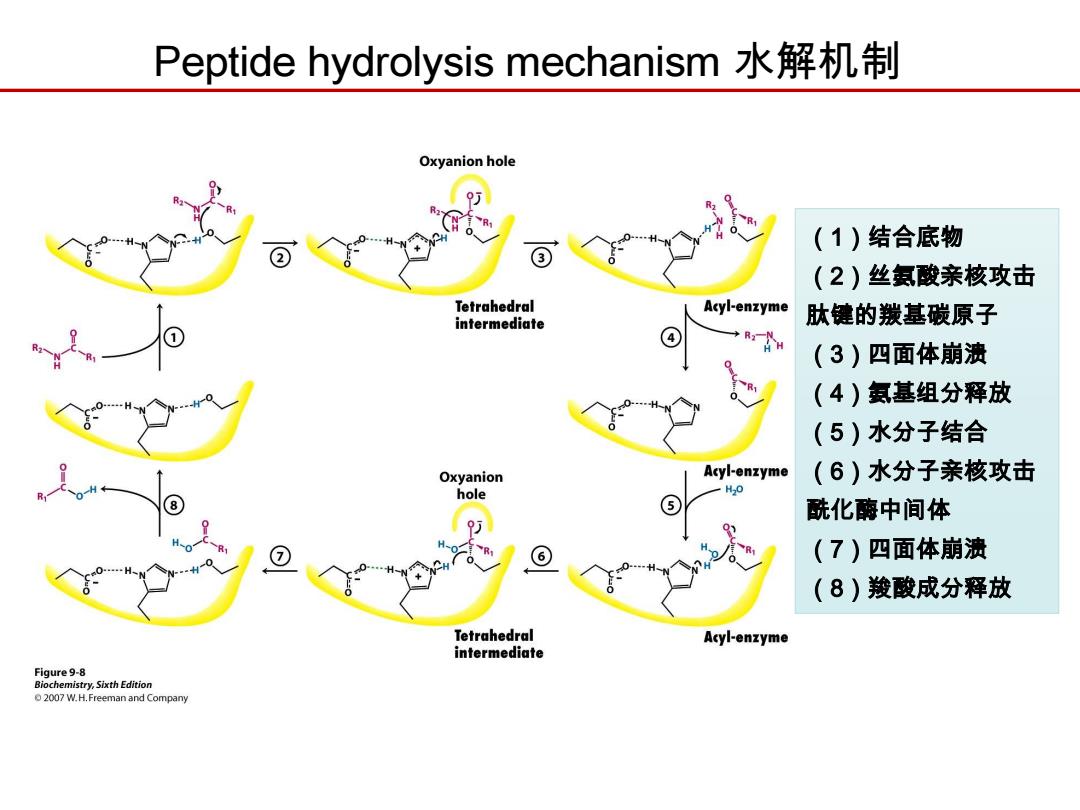
Peptide hydrolysis mechanism水解机制 Oxyanion hole (1)结合底物 3 (2)丝氨酸亲核攻击 Tetrahedral Acyl-enzyme intermediate 肽健的羰基碳原子 (3)四面体崩溃 (4)氨基组分释放 (5)水分子结合 Oxyanion Acyl-enzyme (6)水分子亲核攻击 hole H20 5 酰化酶中间体 (7)四面体崩溃 (8)羧酸成分释放 Tetrahedral Acyl-enzyme intermediate Figure 9-8 Biochemistry,Sixth Edition 2007 W.H.Freeman and Company
Peptide hydrolysis mechanism 水解机制 (1)结合底物 (2)丝氨酸亲核攻击 肽键的羰基碳原子 (3)四面体崩溃 (4)氨基组分释放 (5)水分子结合 (6)水分子亲核攻击 酰化酶中间体 (7)四面体崩溃 (8)羧酸成分释放
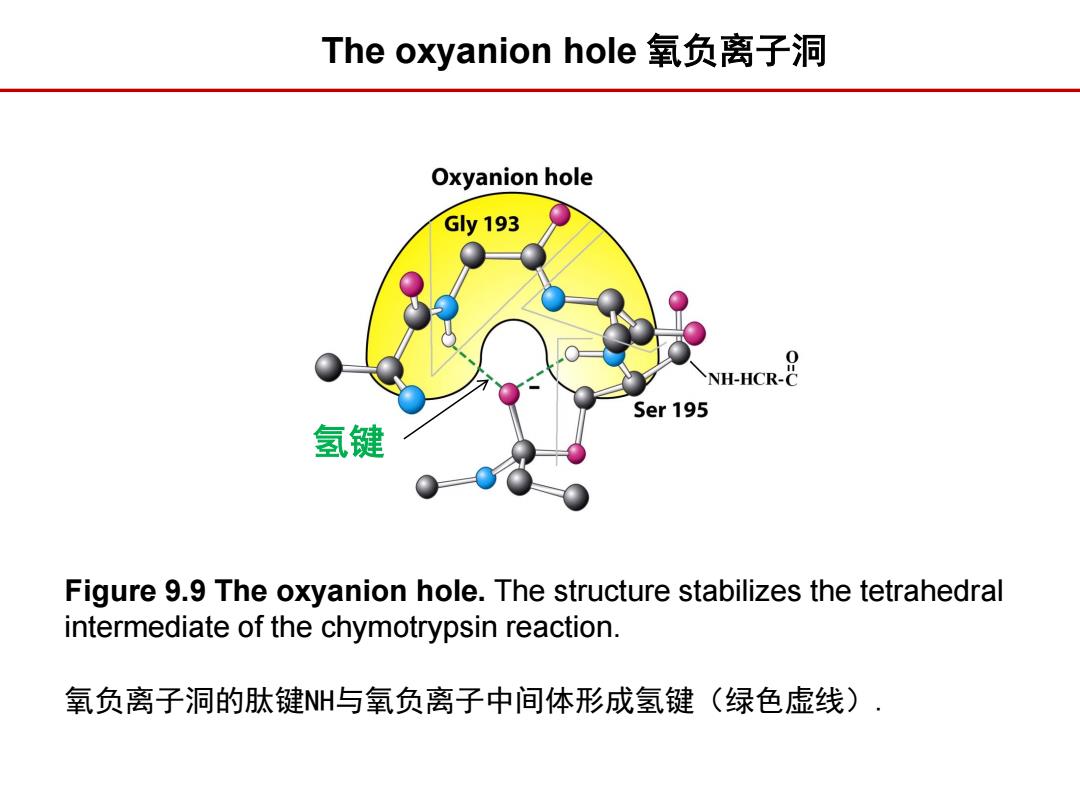
The oxyanion hole氧负离子洞 Oxyanion hole Gy193 NH-HCR- Ser 195 氢键 Figure 9.9 The oxyanion hole.The structure stabilizes the tetrahedral intermediate of the chymotrypsin reaction. 氧负离子洞的肽键NH与氧负离子中间体形成氢键(绿色虚线)
Figure 9.9 The oxyanion hole. The structure stabilizes the tetrahedral intermediate of the chymotrypsin reaction. 氧负离子洞的肽键NH与氧负离子中间体形成氢键(绿色虚线). The oxyanion hole 氧负离子洞 氢键

Substrate Preference底物选择性 Examination of the three-dimensional structure of chymotrypsin with substrate analogs and enzyme inhibitors revealed the presence of a deep, relatively hydrophobic pocket,called the Sl pocket,into which the long, uncharged side chains of residues such as phenylalanine and tryptophan can fit. 酶与底物类似物和抑制剂形成复合物的三维结构,发现酶分子有一个相对疏 水的深口袋,成为$1口袋,适于长的非极性氨基酸侧链。 Figure 9.10 The binding of an appropriate Ser 195 side chain into this pocket positions the adjacent peptide bond into the active site for Trp 215 cleavage.Serine 195 is positioned to cleave er190 the peptide backbone. Met 192 适当侧链与该口袋结合将邻位肽键定位于断裂 Gly 216 Gly 22 位点。胰凝乳蛋白酶的底物特异性几乎完全取 决于肽键N端的氨基酸侧链。 Ser 189 月gure9-10 Blochemistry,Sixth Edition o2007W.H.Freeman and Compamy
Examination of the three-dimensional structure of chymotrypsin with substrate analogs and enzyme inhibitors revealed the presence of a deep, relatively hydrophobic pocket, called the Sl pocket, into which the long, uncharged side chains of residues such as phenylalanine and tryptophan can fit. 酶与底物类似物和抑制剂形成复合物的三维结构,发现酶分子有一个相对疏 水的深口袋,成为S1口袋,适于长的非极性氨基酸侧链。 Substrate Preference 底物选择性 Figure 9.10 The binding of an appropriate side chain into this pocket positions the adjacent peptide bond into the active site for cleavage. Serine 195 is positioned to cleave the peptide backbone. 适当侧链与该口袋结合将邻位肽键定位于断裂 位点。胰凝乳蛋白酶的底物特异性几乎完全取 决于肽键N-端的氨基酸侧链