
3,两种一元弱酸混合酸的滴定 (例〉0.02 mol/L NaOH滴定含0.02mol/LHAc和0.02 mol/L NH,CI的混合液,可否选择滴定,连续滴定?如能 滴,随机误差多大? 解:人w>K;,先滴HAc,化学计量点为Ac和NH4 [H]+[HAc]三NH]+[OH] 近似 [HAcleg [NH3]ea 相当于NH3+HAc=NH+AC滴定反应 K:=INH:I[AC 1 [H']K [NH,]HAc [H']K
〈例〉0.02 mol/L NaOH 滴定含 0.02 mol/L HAc 和 0.02 mol/L NH4Cl 的混合液,可否选择滴定,连续滴定?如能 滴,随机误差多大? 解: + > 4 NH HAc Ka Ka ,先滴HAc,化学计量点为Ac-和 NH4+ [H+] + [HAc] = [NH3] + [OH-] 近似 [HAc]eq = [NH3]eq 相当于 NH 3 + HAc = NH4+ + Ac- 滴定反应 + = = + + − + 4 [ ] [ ] [ ][ ] [ ][ ] 3 4 NH HAc a a t K K H H NH HAc NH Ac K 3. 两种一元弱酸混合酸的滴定
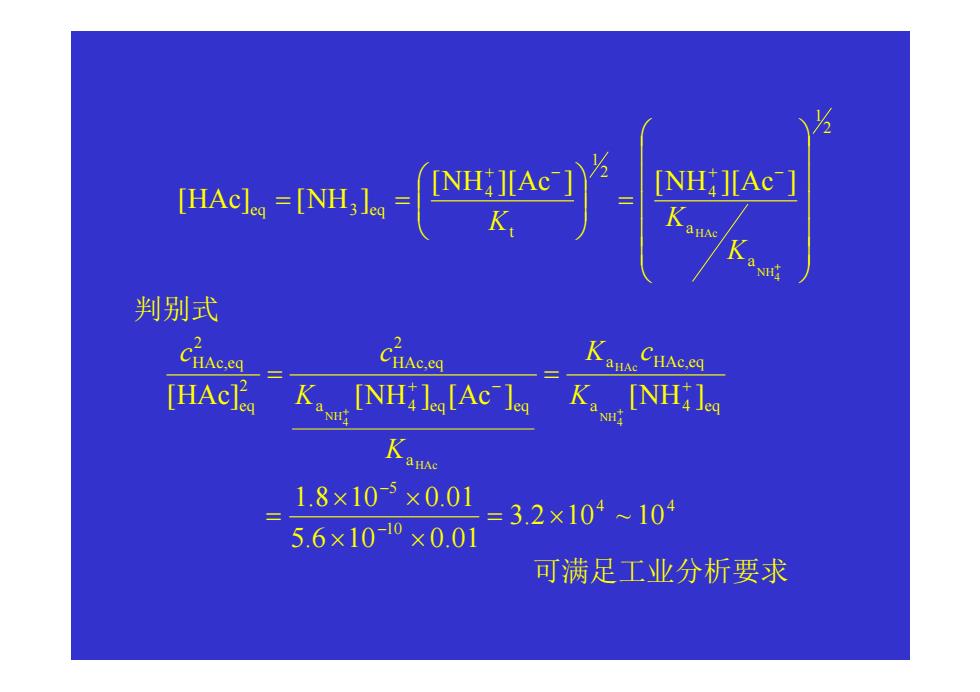
NH 判别式 Cice Cc anAe CHAc.eq [HAc K [NH:lg[Ac lea K[NHi l NH Kanse 1.8×10-×0.01 =3.2×104104 5.6×100×0.01 可满足工业分析要求
2 1 a a 4 2 1 t 4 eq 3 eq 4 NH HAc [NH ][Ac ] [NH ][Ac ] [HAc ] [NH ] = = = + + − + − K K K 判别式 a 4 eq a HAc,eq a a 4 eq eq 2 HAc,eq 2 eq 2 HAc,eq [HAc ] [NH ] [Ac ] [NH ] 4 NH HAc HAc 4 NH + − + + + = = K K c K K c c 4 4 10 5 3.2 10 ~ 10 5.6 10 0.01 1.8 10 0.01 = × × × × × = − − 可满足工业分析要求
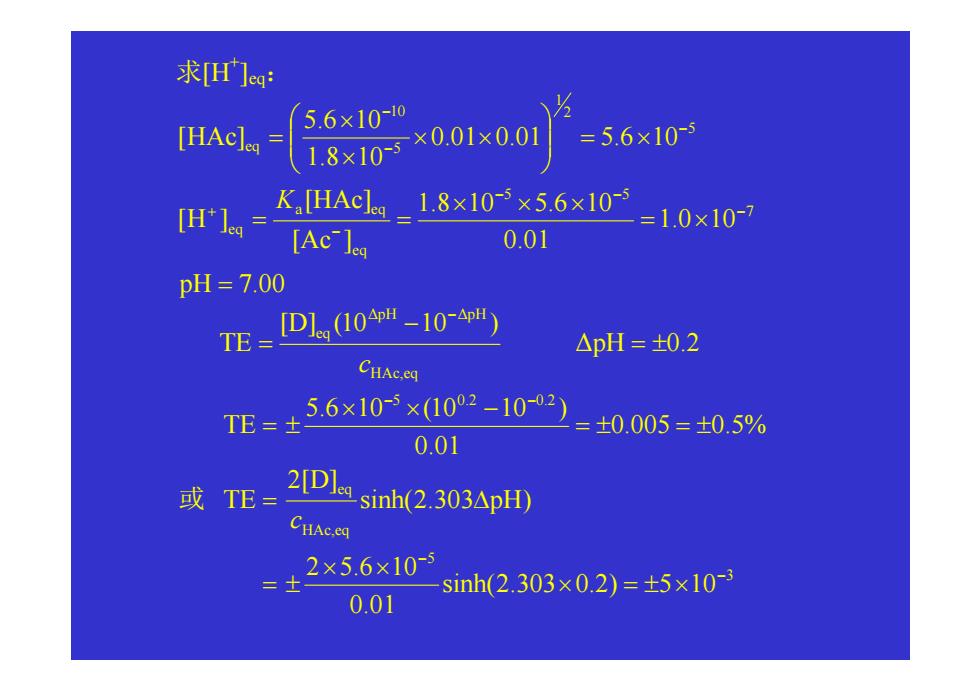
求[H]ea: [HAclea= 5.6×1010 1.8×10 ×0.01×0.01 =5.6×10- [H1= K.[HAcl_18×103×5.6×10 =1.0×107 [Acle 0.01 pH=7.00 TE= D1(10-10p) △pH=t0.2 CHAc.q TE= 56×10-5×(102-10-02 =±0.005=±05% 0.01 或TE= 2[D sinh(2303△pH) CHAc.eq 2×5.6×10 sinh(2303×02)=±5×10 0.01
求[H + ]eq: pH 7.00 1.0 10 0.01 1.8 10 5.6 10 [Ac ] [HAc] [ H ] 0.01 0.01 5.6 10 1.8 10 5.6 10 [HAc ] 7 5 5 eq a eq eq 5 2 1 5 10 eq = = × × × × = = = × × × × × = − − − − + − − − K HAc,eq ∆pH ∆pH eq [D] (10 10 ) TE c − − = ∆pH = ± 0.2 3 5 HAc,eq eq 5 0.2 0.2 sinh( 2.303 0.2 ) 5 10 0.01 2 5.6 10 sinh( 2.303 pH ) 2[D] TE 0.005 0.5 % 0.01 5.6 10 (10 10 ) TE − − − − × = ± × × × = ± = ∆ = ± = ± × × − = ± c 或