
Rb Cs 181 Low melting point Be Mg a Sr Ba Li Na KRb Cs Fr
Be Mg Ca Sr Ba Rb Cs Low melting point Li Na K Rb Cs Fr

2.Chemical properties: ·React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. The elementary substances form the corresponding oxides when they burn in air: LiO Na202 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 Na,O2 Li,C Pale yellow K Pale yellow Magnesium burning in air
The elementary substances form the corresponding oxides when they burn in air: Li2O Na2O2 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 • React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. 2.Chemical properties: Magnesium burning in air Li2O Na2O2 KO2 Pale yellow Pale yellow

.React with water 2M+2H,O-2MOH+H2(g) Li Na K Bromothymol blue indicator 溴百里酚兰指示剂 Ca
•React with water Li Na K Ca 2M + 2H 2O → 2MOH + H 2(g) Bromothymol blue indicator 溴百里酚兰 指示剂
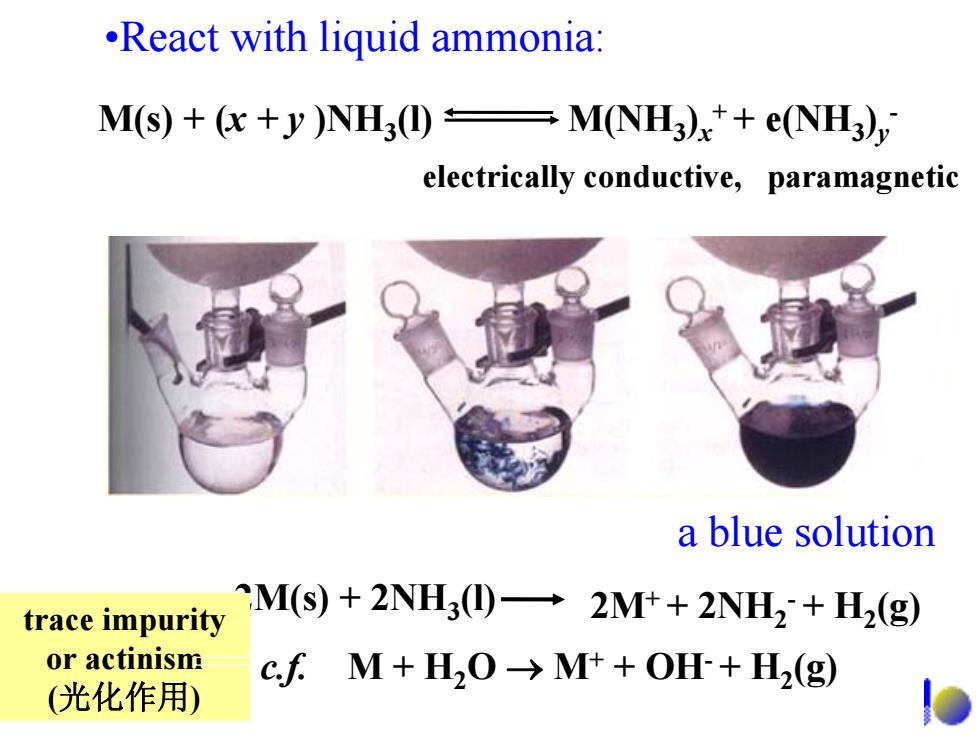
.React with liquid ammonia: M(s)+(x+y)NH3(I)M(NH3)x++e(NH3), electrically conductive,paramagnetic a blue solution trace impurity CM(s)+2NH3(I)-2M++2NH2+H2(g) or actinism cfM+H2O→Mt+OH+H2(g) (光化作用)
•React with liquid ammonia: a blue solution 2M(s) + 2NH 3(l) 2M+ + 2NH 2 - + H 2(g) c.f. M + H 2O → M + + OH - + H 2(g) M(s) + (x + y )NH 3(l) M(NH 3 ) x + + e(NH 3 )y - electrically conductive, paramagnetic trace impurity or actinism (光化作用 )