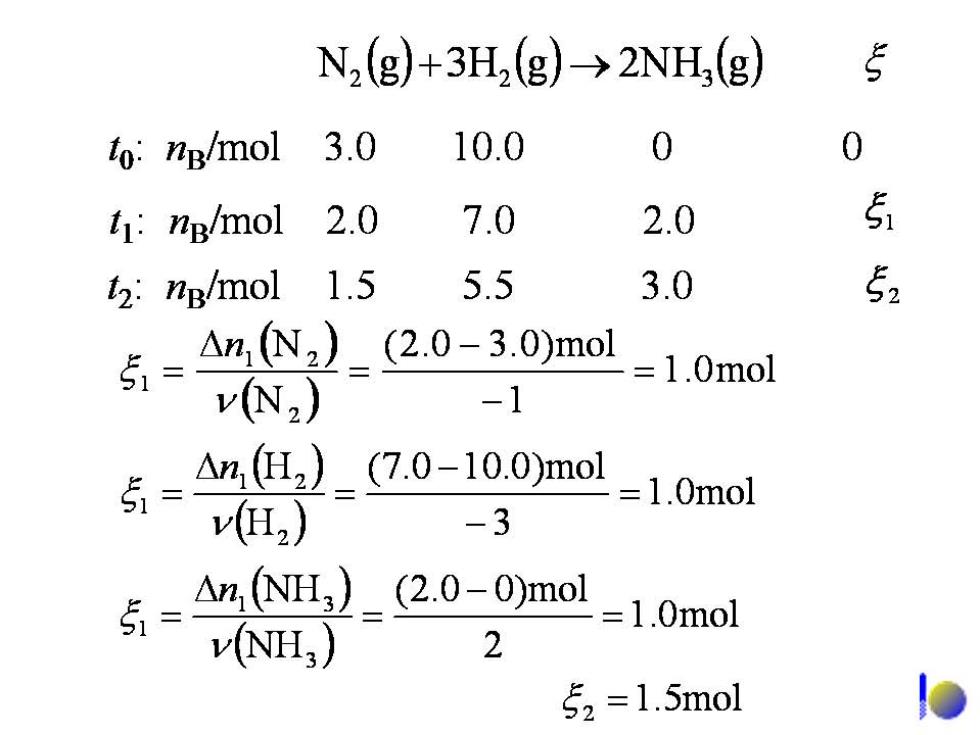
N2(g)+3H2(g)→2NH,(g to:ng/mol 3.0 10.0 0 0 t1:ng/mol 2.0 7.0 2.0 5 2:ng/mol 1.5 5.5 3.0 52 5有 An,N2)_(2.0-3.0)mo v(N2) =1.0mol -1 △n,(I2)_(7.0-10.0)mol v2) -3 =1.0mo1 △n,NH3)_(2.0-0)mol vNH) 2 =1.0mol 52=1.5mol D
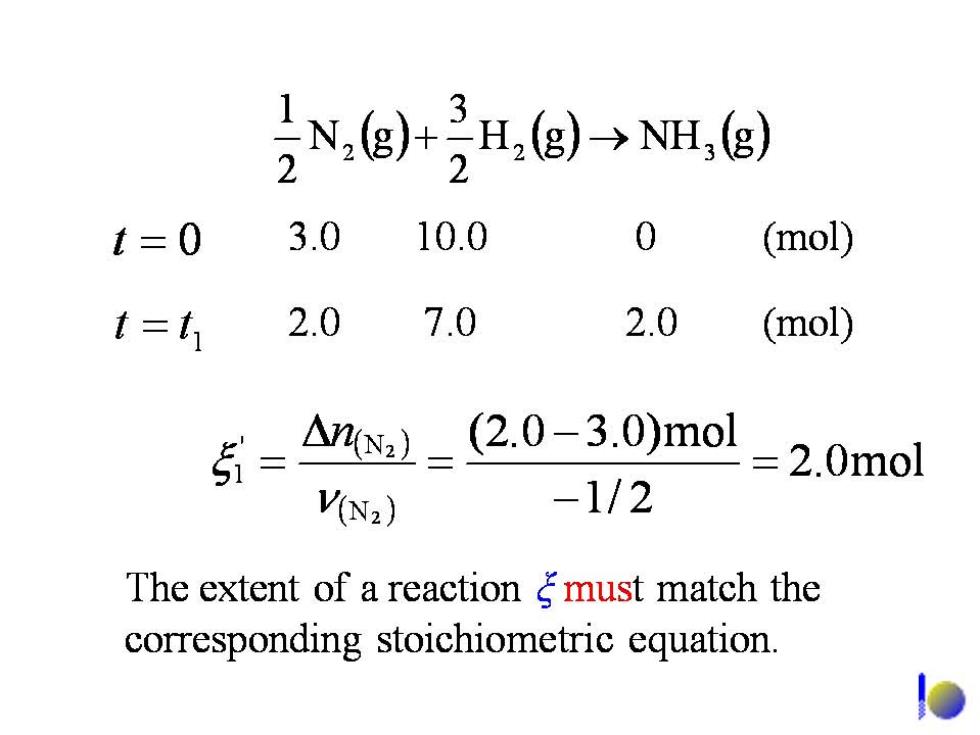
,N,g)+H,g)-→NH,(g) t=0 3.0 10.0 0 (mol) t=1 2.0 7.0 2.0 (mol) 分-A-2.0-30)mol 2 .0mol V(N2) -1/2 The extent of a reaction gmust match the corresponding stoichiometric equation

2.2 the First law of thermodynamics 2.2.1 Heat and work 2.2.2 Thermodynamic energy 2.2.3 the First law of thermo- dynamics