Contents of Today S.J.T.U. Phase Transformation and Applications Review previous Condition of equilibrium Phase equilibrium Clapeyron equation in vapor equilibria Variation of vapor pressure with particle size Second-order transition SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Contents of Today Review previous Condition of equilibrium Phase equilibrium Clapeyron equation in vapor equilibria Variation of vapor pressure with particle size Second-order transition
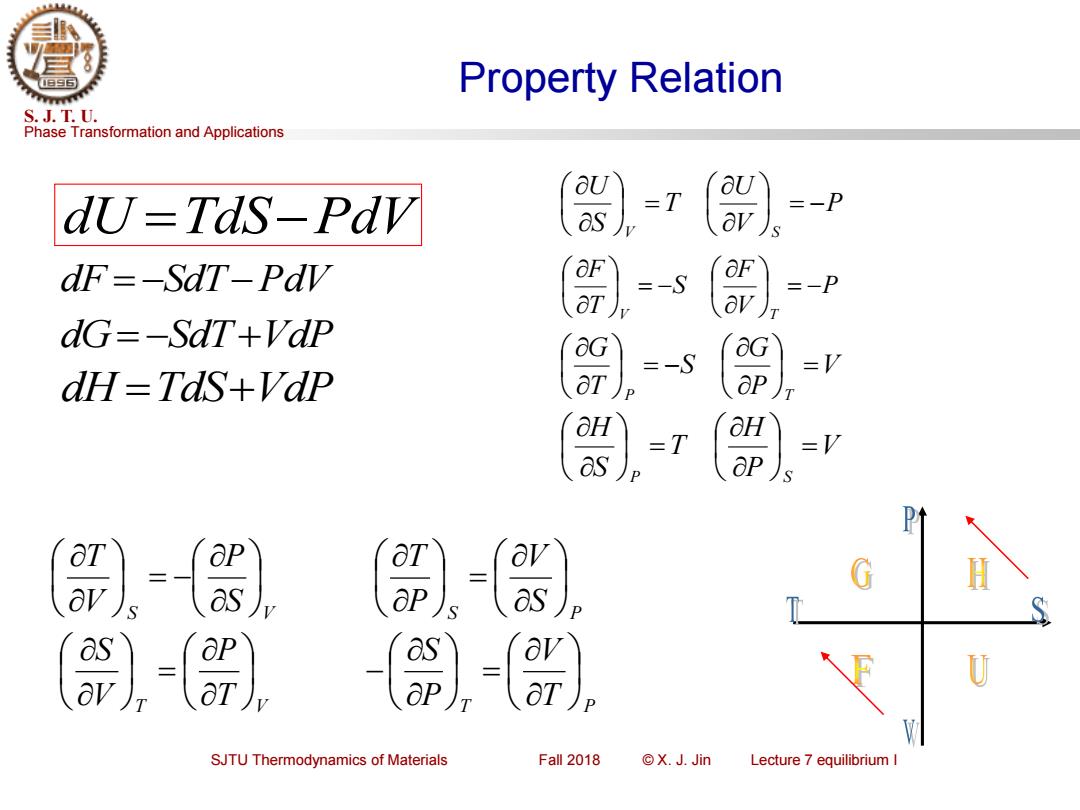
Property Relation S.J.T.U. Phase Transformation and Applications aU dU-Tds-Pdv =T =-P dF=-SdT-Pav =-S 8) dG=-SdT+Vdp =-S =V dH=TaS+Vap -T =V 二 as ap) H as aP as ap SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Property Relation S S V P V T = − S S P V P T = T T V P V S = T T P V P S = − dU =TdS−PdV dF = −SdT−PdV dG= −SdT+VdP dH =TdS+VdP P V U T S U V S = − = P V F S T F V T = − = − V P G S T G P T = = − V P H T S H P S = =
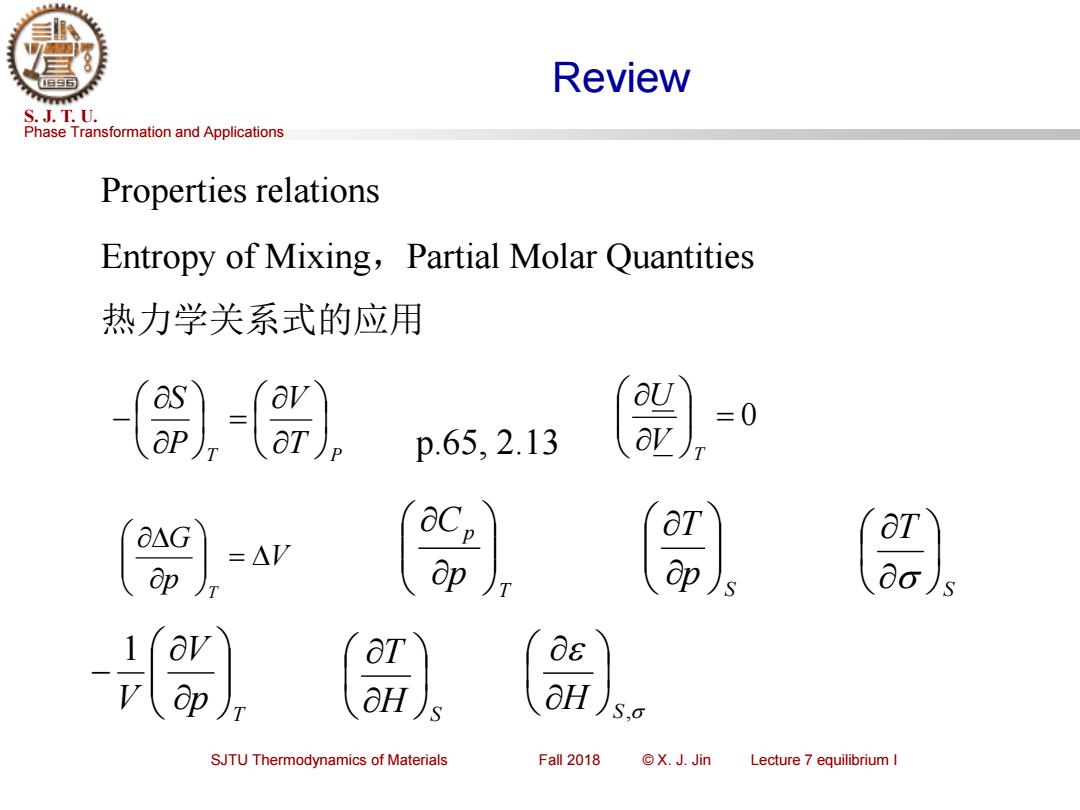
Review S.J.T.U. Phase Transformation and Applications Properties relations Entropy of Mixing,Partial Molar Quantities 热力学关系式的应用 -) p.65..13 a△G =△V ap ap (av 0s OH aH S,o SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Review Properties relations Entropy of Mixing,Partial Molar Quantities 热力学关系式的应用 T T P V P S = − = 0 T V U p.65, 2.13 V p G T = T p p C S p T S T T p V V − 1 H S T H S,

Quiz Q3 Answer S.J.T.U. Phase Transformation and Applications 15.进行下述过程时,系统的△U、△H、△S和△G何者为零? (1)非理想气体的卡诺循环; (2)隔离系统中的任意过程: (3)在100℃,01325Pa下1mol水蒸发成水蒸气; (4)绝热可逆过程。 15题:1)内能变化为0,焓变为0,熵变为0,自由能变化为0。 2):内能变化为0, 焓变不一定为0,熵变不一定为0,自由能变化不 一定为0。 3)自由能变化为0。错,主要认为相变过程中内能为0,焓变也为0。 4)只有熵变为0。错误很多。 SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Quiz Q3 Answer 15题:1)内能变化为0,焓变为0,熵变为0,自由能变化为0。 2):内能变化为0,焓变不一定为0,熵变不一定为0,自由能变化不 一定为0。 3)自由能变化为0。错,主要认为相变过程中内能为0,焓变也为0。 4)只有熵变为0。错误很多
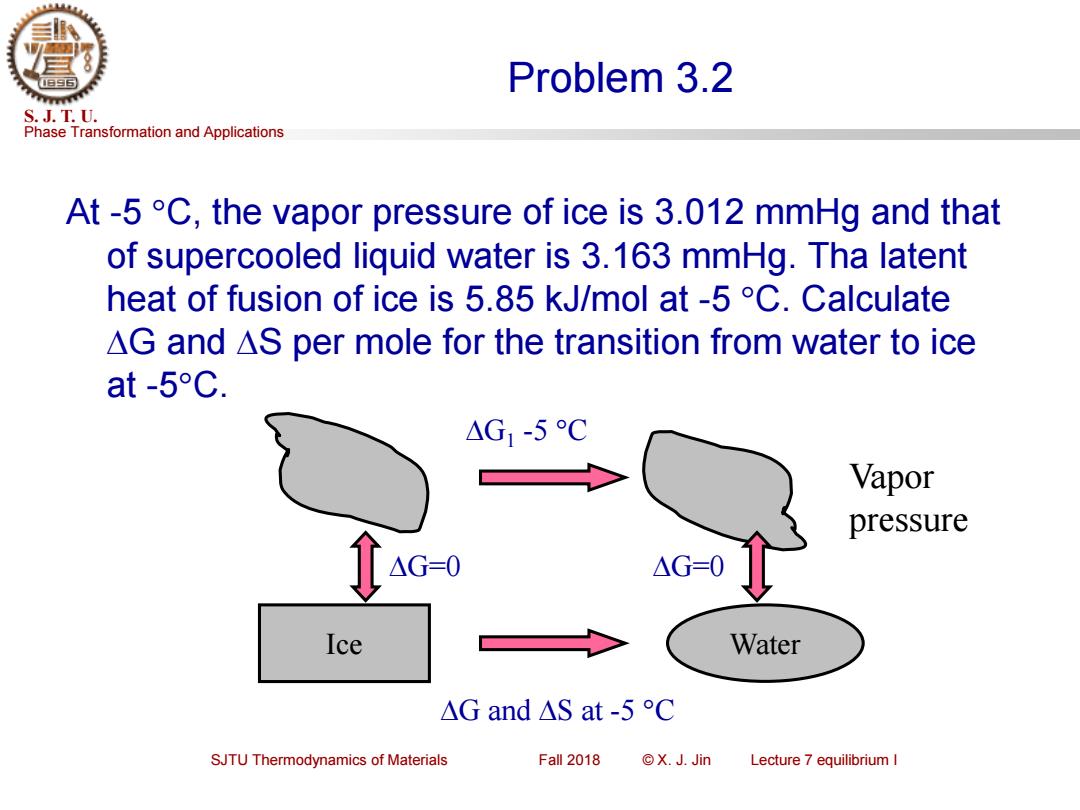
Problem 3.2 S.J.T.U. Phase Transformation and Applications At-5 C,the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg.Tha latent heat of fusion of ice is 5.85 kJ/mol at -5C.Calculate AG and AS per mole for the transition from water to ice at-5°C. △G1-5C Vapor pressure △G=0 △G=-0 Ice Water △Gand△Sat-5C SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium I
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Problem 3.2 At -5 C, the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg. Tha latent heat of fusion of ice is 5.85 kJ/mol at -5 C. Calculate G and S per mole for the transition from water to ice at -5C. Ice Water G and S at -5 C G1 -5 C G=0 G=0 Vapor pressure