Contents of Today S.J.T.U. Phase Transformation and Applications Review previous Property relation Functions F and G Partial molar quantities Property relation derived from U,H,F,and G etc. SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 6 Property Relation II Contents of Today Review previous Property relation Functions F and G Partial molar quantities Property relation derived from U, H, F, and G etc
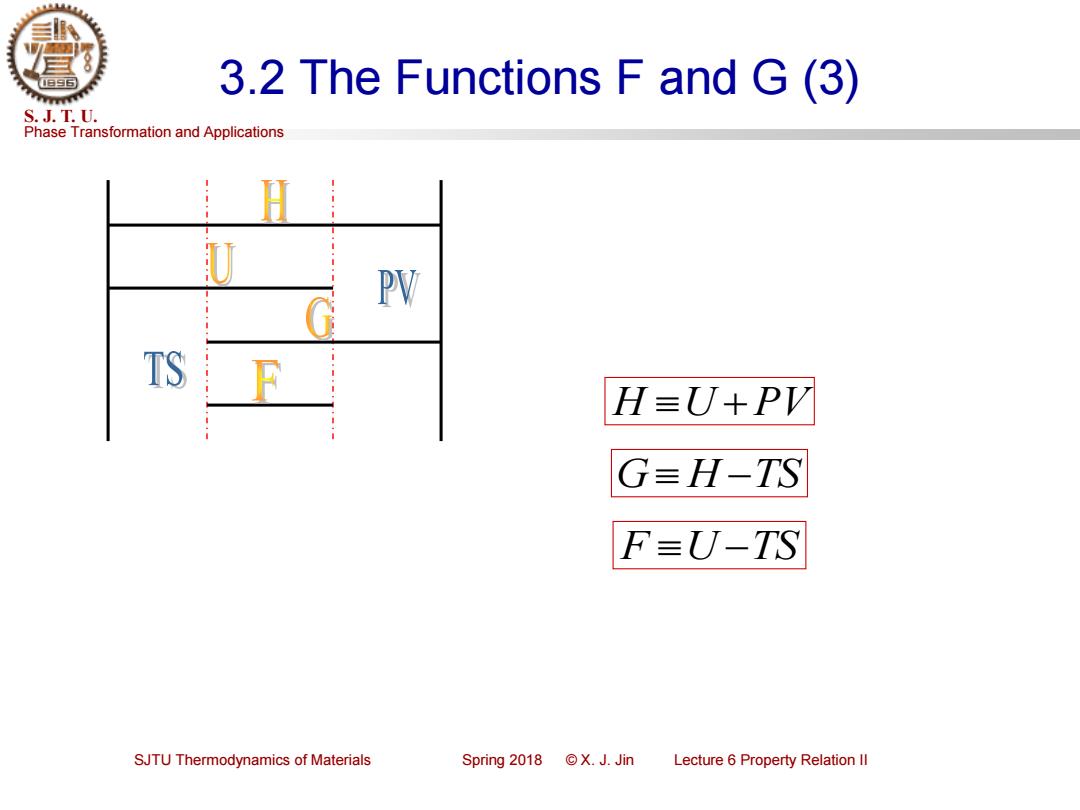
3.2 The Functions F and G(3) S.J.T.U. Phase Transformation and Applications U PV TS H≡J+PV G=H-TS F=U-TS SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 6 Property Relation II 3.2 The Functions F and G (3) F U −TS G H −TS H U +PV
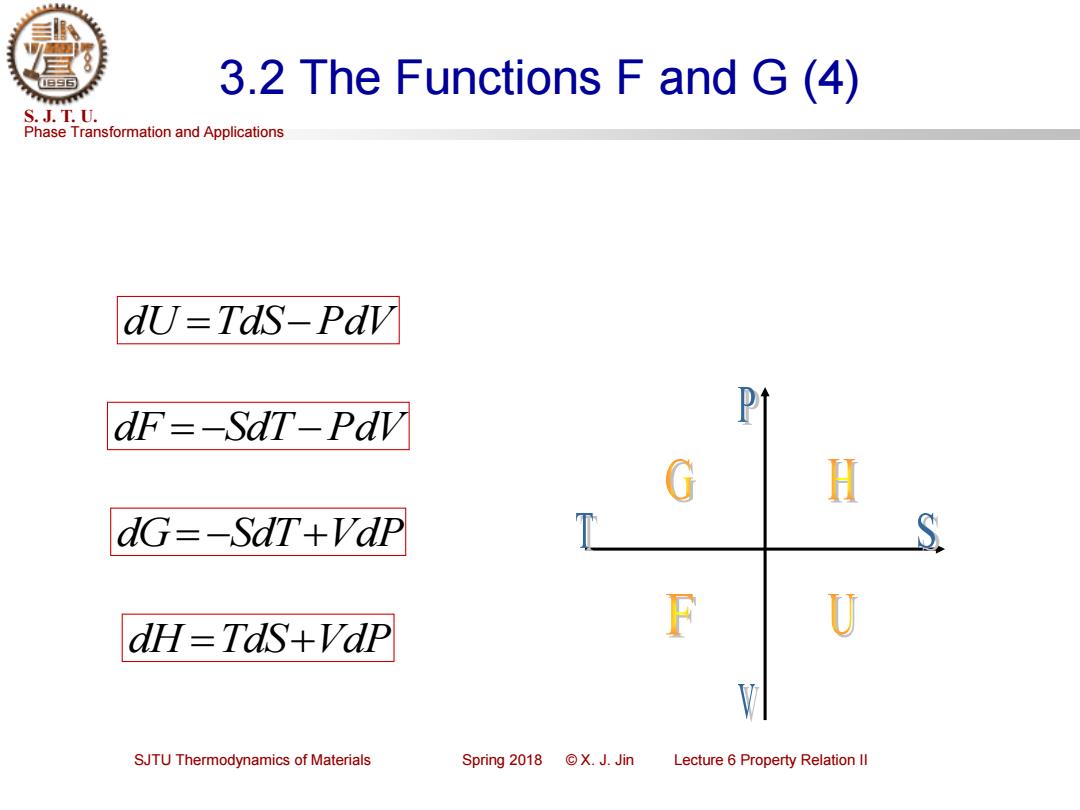
3.2 The Functions F and G(4) S.J.T.U. Phase Transformation and Applications dU=Tds-Pav dF=-SdT-Pdv Pt H dG=-SdT+VdP S dH TdS+Vdp F U SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 6 Property Relation II 3.2 The Functions F and G (4) dU =TdS−PdV dF = −SdT−PdV dG= −SdT+VdP dH =TdS+VdP
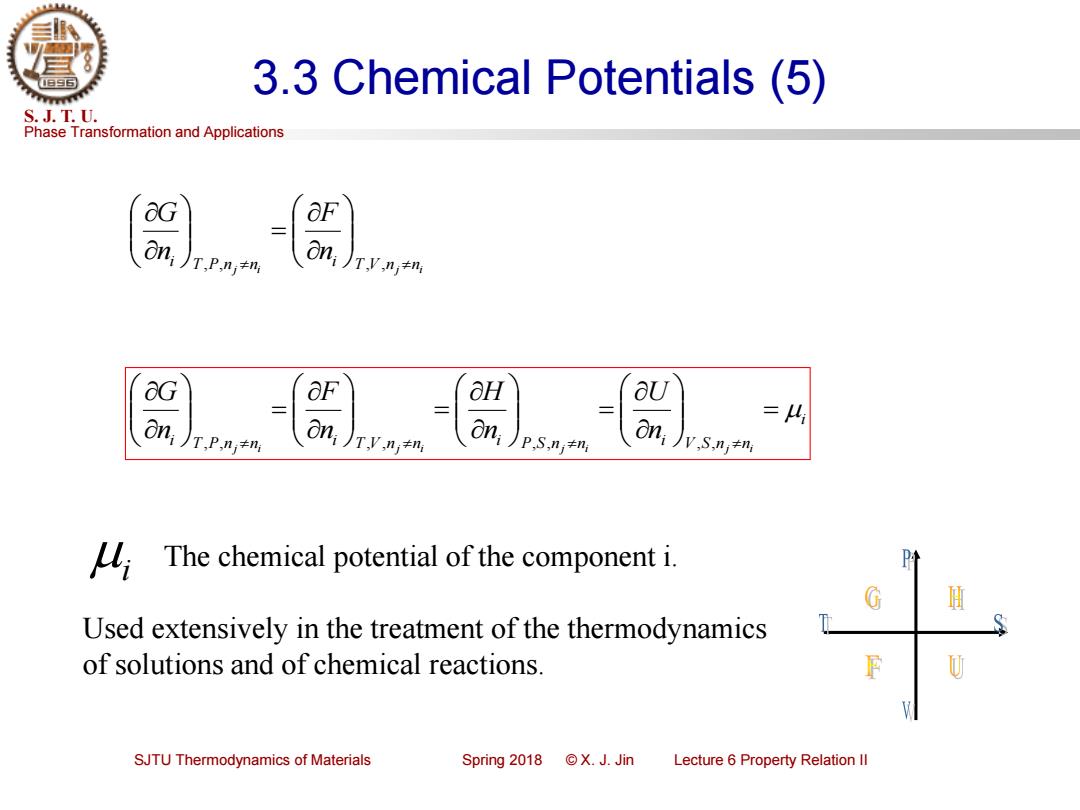
3.3 Chemical Potentials (5) S.J.T.U. Phase Transformation and Applications &G On T,P,nj≠n G OF aH aU on; on =4 T,P.nj+ni oni),+m P.S,ni+ni V.S.n;+ni The chemical potential of the component i. G H Used extensively in the treatment of the thermodynamics of solutions and of chemical reactions. SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 6 Property Relation Il
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 6 Property Relation II 3.3 Chemical Potentials (5) j i T V nj ni i T P n n ni F n G = , , , , i i T P nj ni i T V nj ni i P S nj ni i V S nj ni n U n H n F n G = = = = , , , , , , , , i The chemical potential of the component i. Used extensively in the treatment of the thermodynamics of solutions and of chemical reactions
Review S.J.T.U. Phase Transformation and Applications 当系统由始态经过某一过程变到终态Ⅱ后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 H=U+PV dU=TdS-Pdv PW G=H-TS dF=-SaT-Pav TS F=U-TS dG=-SaT+Vdp G H dH=TaS+Vdp U The chemical potential of the component i. &G OF aH aU Oni T,P,n≠n On, On O =4 i )p.S.nj+m i Jv S,nj+m SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 6 Property Relation Il
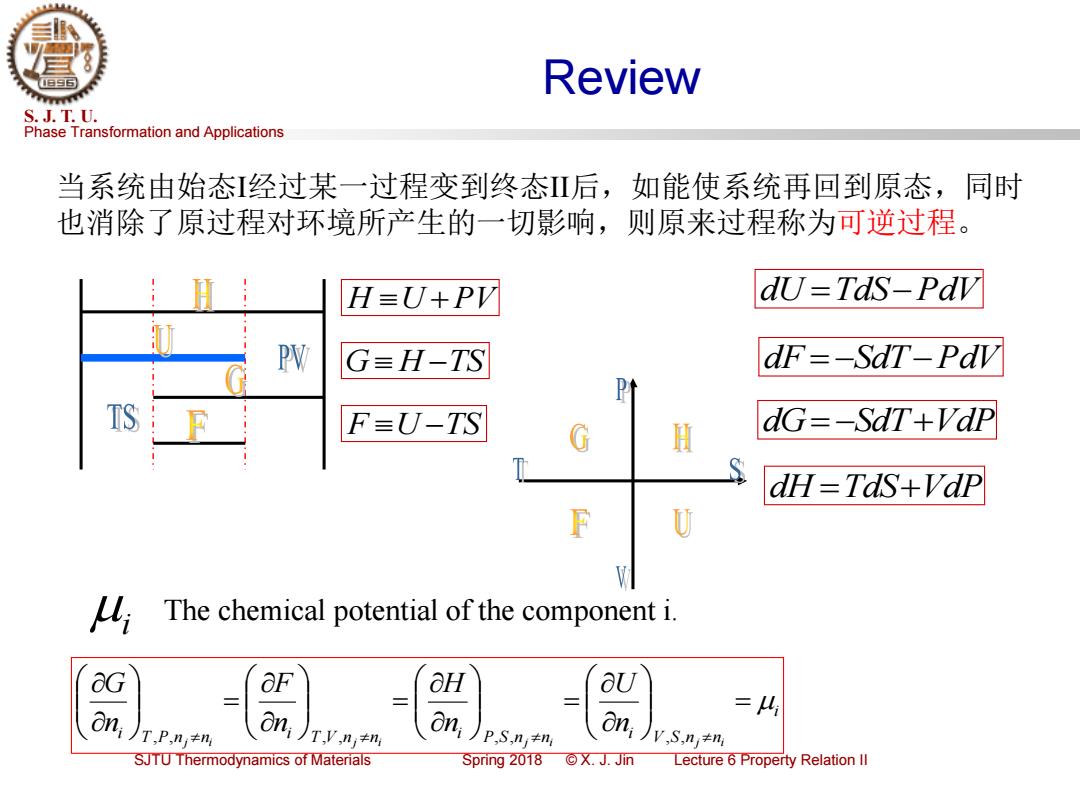
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 6 Property Relation II Review F U −TS G H −TS H U +PV 当系统由始态I经过某一过程变到终态II后,如能使系统再回到原态,同时 也消除了原过程对环境所产生的一切影响,则原来过程称为可逆过程。 dU =TdS−PdV dF = −SdT−PdV dG= −SdT+VdP dH =TdS+VdP i i T P nj ni i T V nj ni i P S nj ni i V S nj ni n U n H n F n G = = = = , , , , , , , , i The chemical potential of the component i