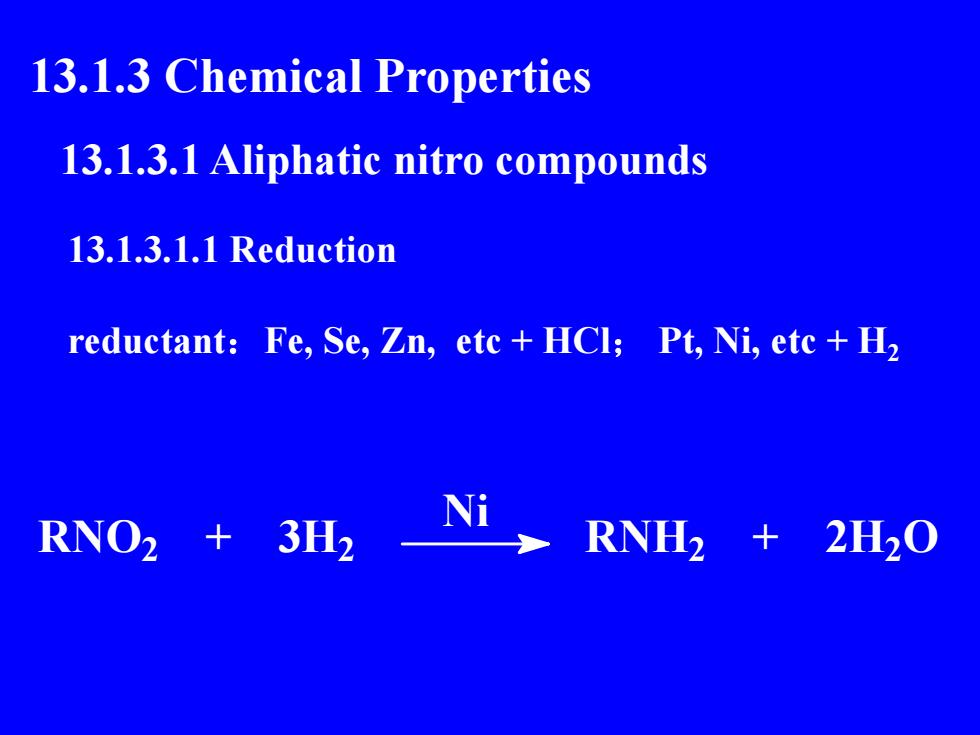
13.1.3 ChemicalProperties13.1.3.1 Aliphatic nitro compounds13.1.3.1.1 Reductionreductant: Fe, Se, Zn, etc +HCl; Pt, Ni, etc +H,NRNO23H2RNH22H20
13.1.3 Chemical Properties 13.1.3.1 Aliphatic nitro compounds 13.1.3.1.1 Reduction reductant:Fe, Se, Zn, etc + HCl; Pt, Ni, etc + H2 RNO2 + 3H2 Ni RNH2 + 2H2O
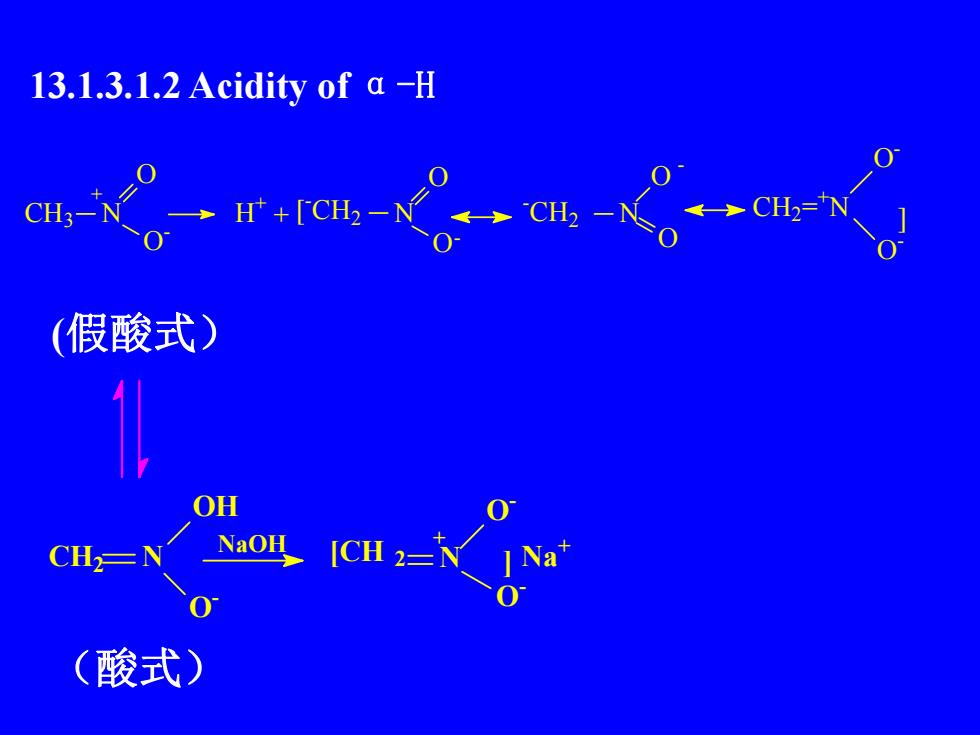
13.1.3.1.2 Acidity of a -HCH2=H+[CH2CH3-CH2O浴(假酸式)OHNaOHJCH 2=CHNa0(酸式)
13.1.3.1.2 Acidity of α-H (假酸式) (酸式) CH2 N OH O - [CH 2 N + O - O - ] Na NaOH + CH3 N O O - + H + + [ -CH2 N O -CH2 N O O O CH2= +N O - O -] - -
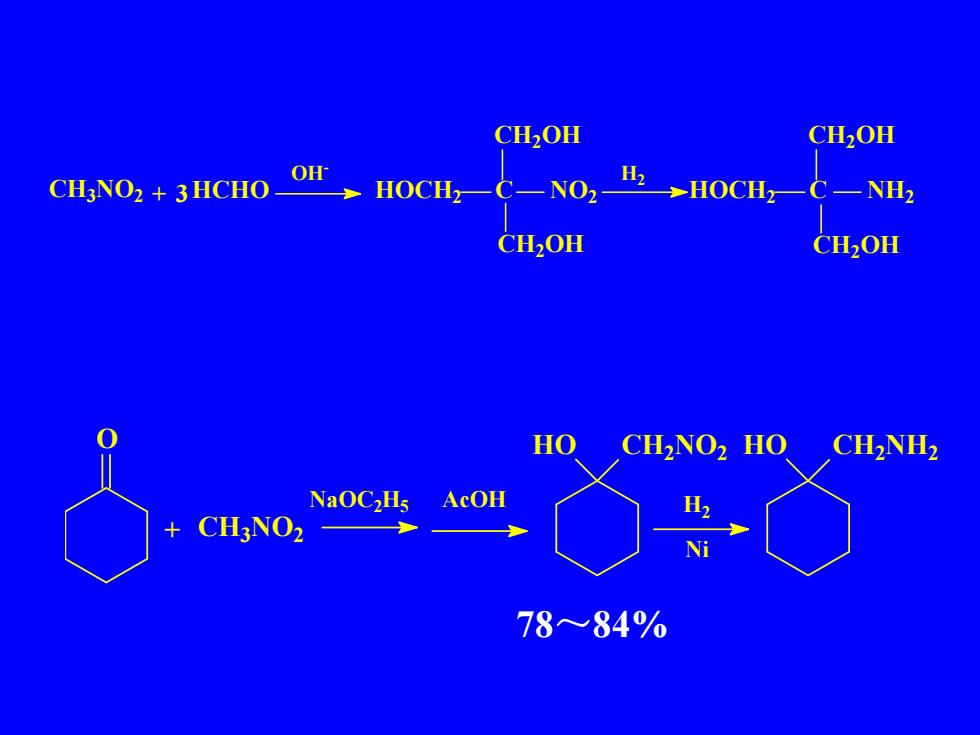
CH,OHCH,OHOHHNO2CH3NO2+3HCHOHOCH,-HOCH2NH2CH,OHCH,OHHOCH,NO2HOCH2NH2NaoC,HsAcOHH2CH,NO2一+Ni78~84%
78~84% CH3NO2 + HCHO OH- HOCH2 C NO2 CH2OH CH2OH H2 HOCH2 C NH2 CH2OH CH2OH 3 O + CH3NO2 NaOC2H5 AcOH HO CH2NO2 HO CH2NH2 H2 Ni
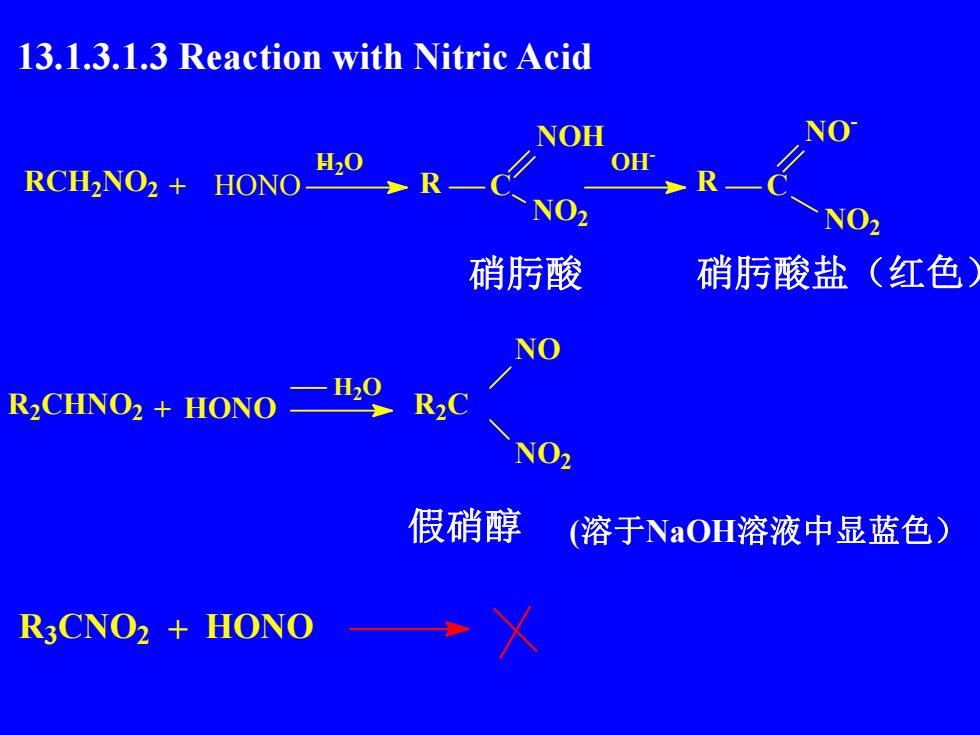
13.1.3.1.3 Reaction with Nitric AcidNONOHH,0OHRCH,NO2+HONONO2NO2硝酸硝酸盐(红色NOH20R2CR,CHNO2 + HONONO2假硝醇(溶于NaOH溶液中显蓝色)R3CNO2 + HONO
13.1.3.1.3 Reaction with Nitric Acid 硝肟酸 硝肟酸盐(红色) 假硝醇 (溶于NaOH溶液中显蓝色) R3CNO2 + HONO RCH2NO2 + HONO H- 2O R C NO2 NOH OH- R C NO - NO2 R2CHNO2 + HONO H2O R2C NO NO2
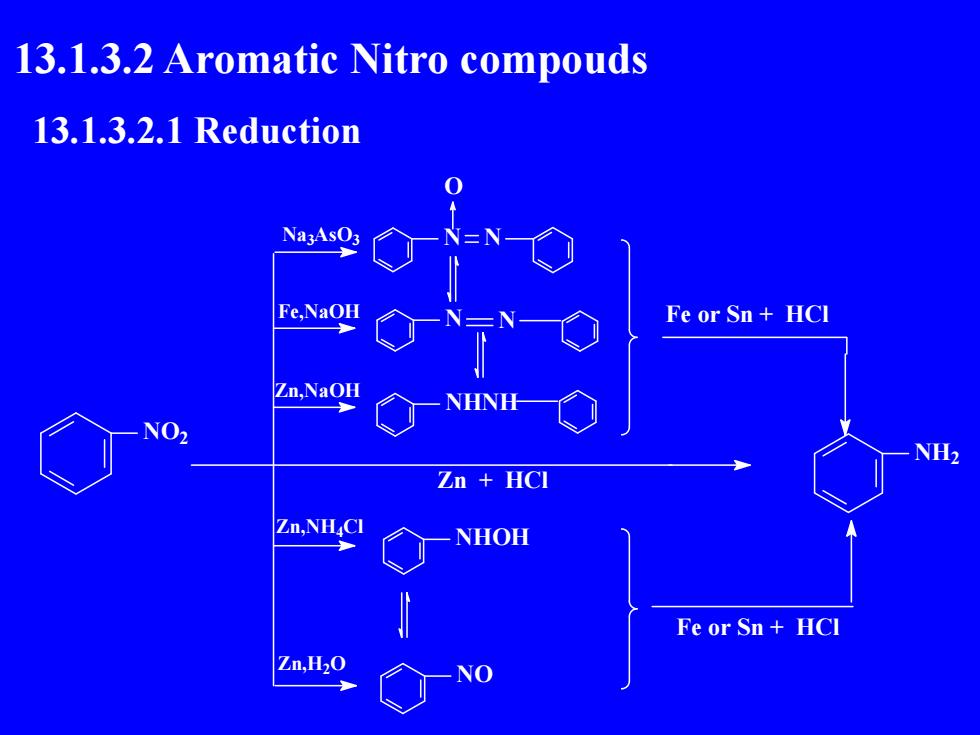
13.1.3.2 Aromatic Nitro compouds13.1.3.2.1ReductionNa3AsO3Fe,NaOHFe or Sn+ HCIZn,NaOHNHNHNO2NH2Zn+ HCIZn,NH.CNHOHFeor Sn+ HCIZn,H,0NO
13.1.3.2 Aromatic Nitro compouds 13.1.3.2.1 Reduction NO2 Na3AsO3 Fe,NaOH Zn,NaOH Zn,NH4Cl Zn,H2O N N O N N NHNH NHOH NO NH2 Fe or Sn + HCl Fe or Sn + HCl Zn + HCl