
Preparation of Alkyl Halides (CHa)COH+HCI 25C(CHa)CCI+H2O 78-88% 80-100°C OH HBr Br+H2O 73% CHs(CH2)sCH2OH HBr 120C CH3(CH2)5CH2Br H2O 87-90%

Preparation of Alkyl Halides A mixture of sodium bromide and sulfuric acid may be used in place of HBr. NaBr H2SO4 CHCH2CH2CH2OH CH2CH2CH,CH2Br heat 70-83% 120°C CH3(CH2)sCH2OH HBr CH3(CH2)5CH2Br H2O 87-90%
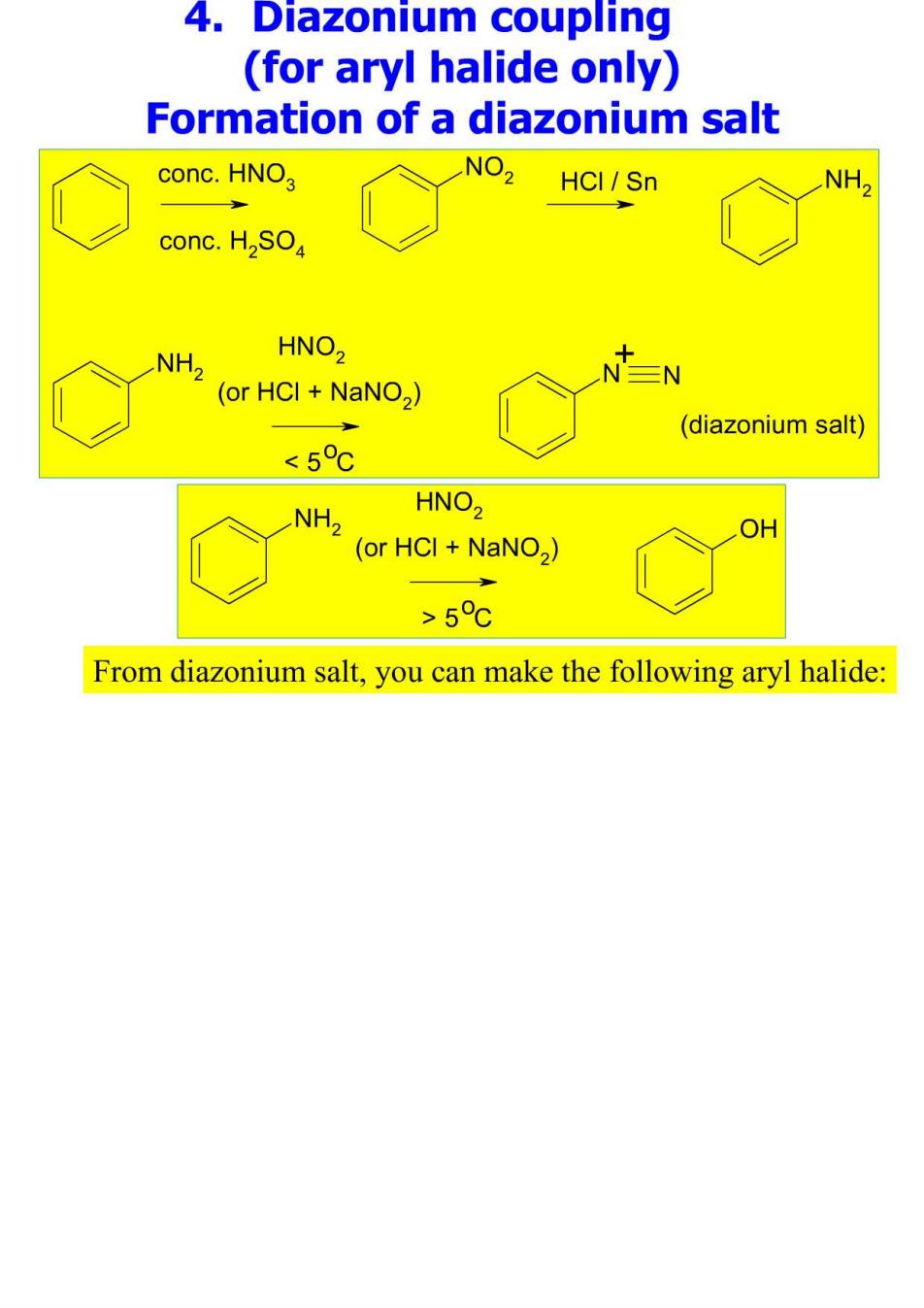
4. Diazonium coupling (for aryl halide only) Formation of a diazonium salt conc.HNO, .NO2 HCI/Sn NH, conc.H2SO NH, HNO, (or HCI+NaNO2) (diazonium salt) <5c NH2 HNO2 (or HCI NaNO2) OH >5℃ From diazonium salt,you can make the following aryl halide:
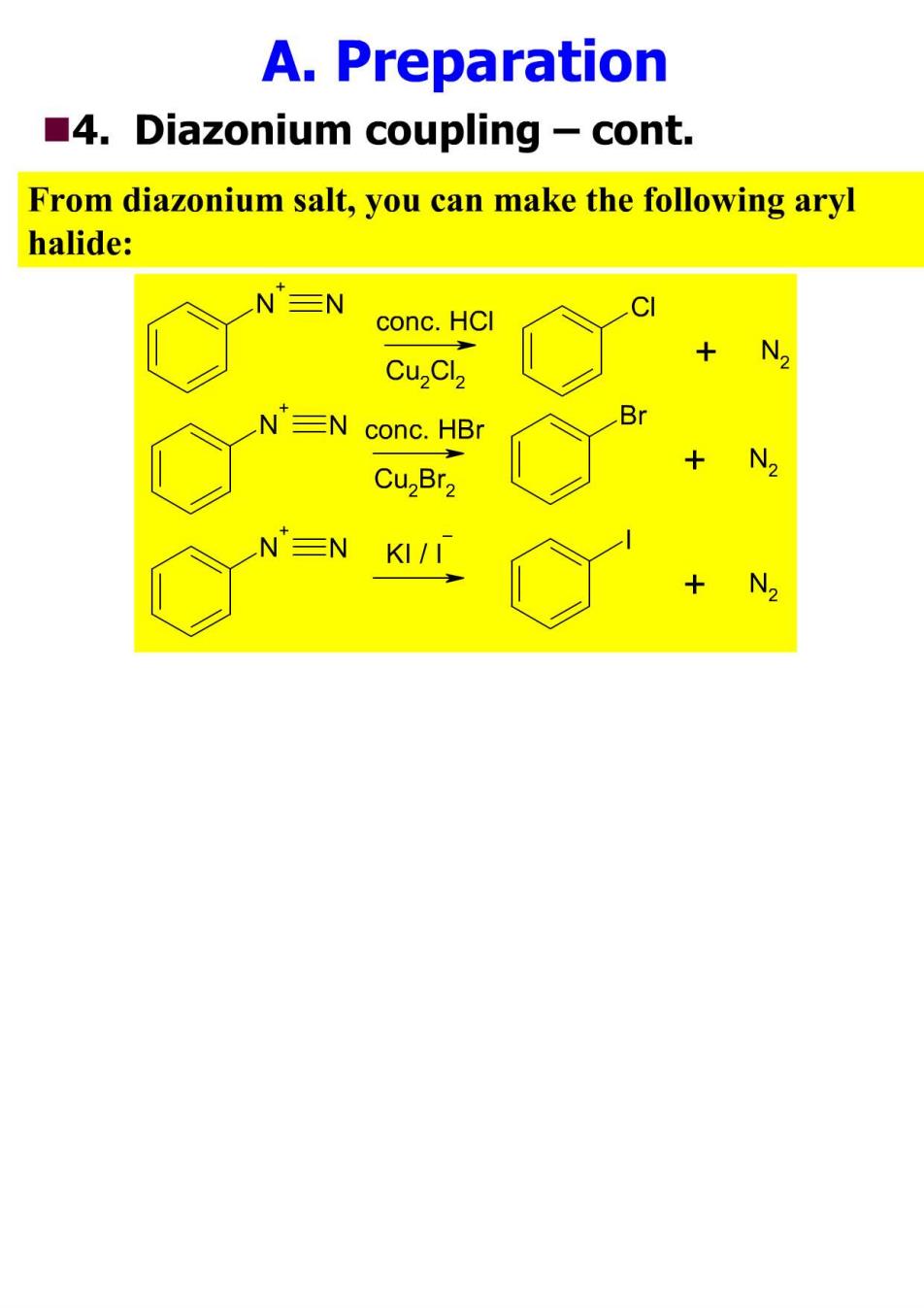
A.Preparation 4.Diazonium coupling -cont. From diazonium salt,you can make the following aryl halide: N三N CI conc.HCI N CuzCl2 N 三N conc.HB N Cu,Br2 N三N KI/I
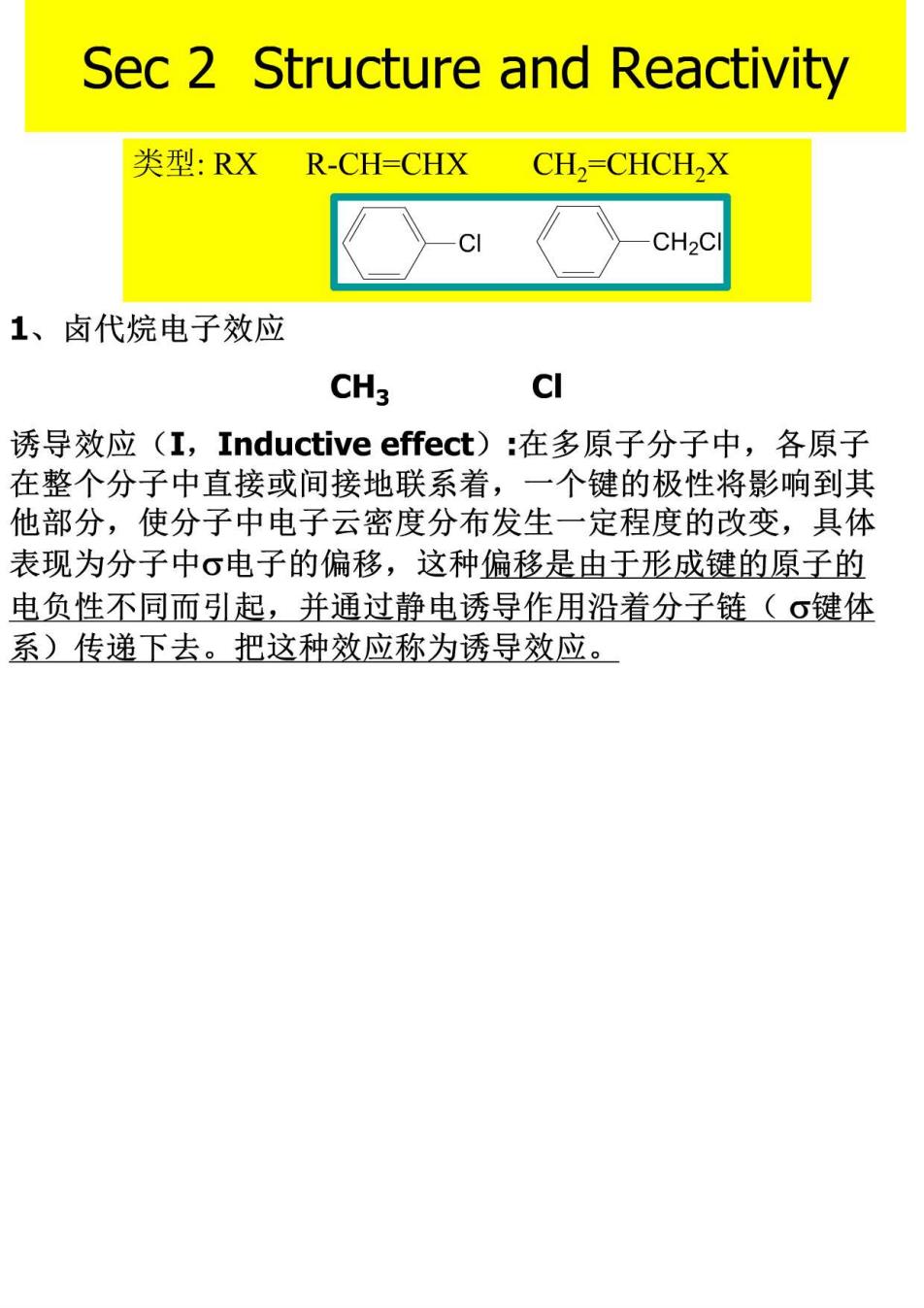
Sec 2 Structure and Reactivity 类型:RXR-CH=CHX CH,=CHCH,X C CH2C 1、卤代烷电子效应 CH3 CI 诱导效应(I,Inductive effect):在多原子分子中,各原子 在整个分子中直接或间接地联系着,一个键的极性将影响到其 他部分,使分子中电子云密度分布发生一定程度的改变,具体 表现为分子中σ电子的偏移,这种偏移是由于形成键的原子的 电负性不同而引起,并通过静电诱导作用沿着分子链(σ键体 系)传递下去。把这种效应称为诱导效应