Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 17 e 18 Aromatic chemistry Key Notes Aromaticity;Resonance Structure;Huckelrule; Electrophilic substitutions;Deactivating group; By Junru Wang Email:wangjr07@163.com 西北农林科技大学理学院
Chapter 17 & 18 Aromatic chemistry Organic Chemistry, 6th Edition L. G. Wade, Jr. By Junru Wang Email: wangjr07@163.com 西北农林科技大学理学院 Key Notes Aromaticity; Resonance Structure; Hückel rule; Electrophilic substitutions; Deactivating group;
CONTENT Structure Physical Properties Aromaticity reactivity Hiickel rule Preparation of Ar compounds Electrophilic substitutions Effects of substituents on E.S. -Synthesis of mono-,di-and tri-substituted benzenes Other reactions -Oxidation and reduction -Nucleophilic Substitution
CONTENT ❖Structure & Physical Properties ❖Aromaticity & reactivity ◼Hückel rule ❖Preparation of Ar compounds ❖Electrophilic substitutions ❖Effects of substituents on E.S. ◼Synthesis of mono-, di- and tri-substituted benzenes ❖Other reactions ◼Oxidation and reduction ◼Nucleophilic Substitution
Sec 1 Structure Physical Properties Discovery of Benzene -Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. -Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6. -Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic
Sec 1 Structure & Physical Properties ❖Discovery of Benzene ◼Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. ◼Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6 . ◼Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic

AdGif-UNREGIST Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order=1 butadiene combined representation Copyright2010 Pearson Prentice Hall,Inc
KekuléStructure ❖Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. ❖Failed to explain existence of only one isomer of 1,2-dichlorobenzene
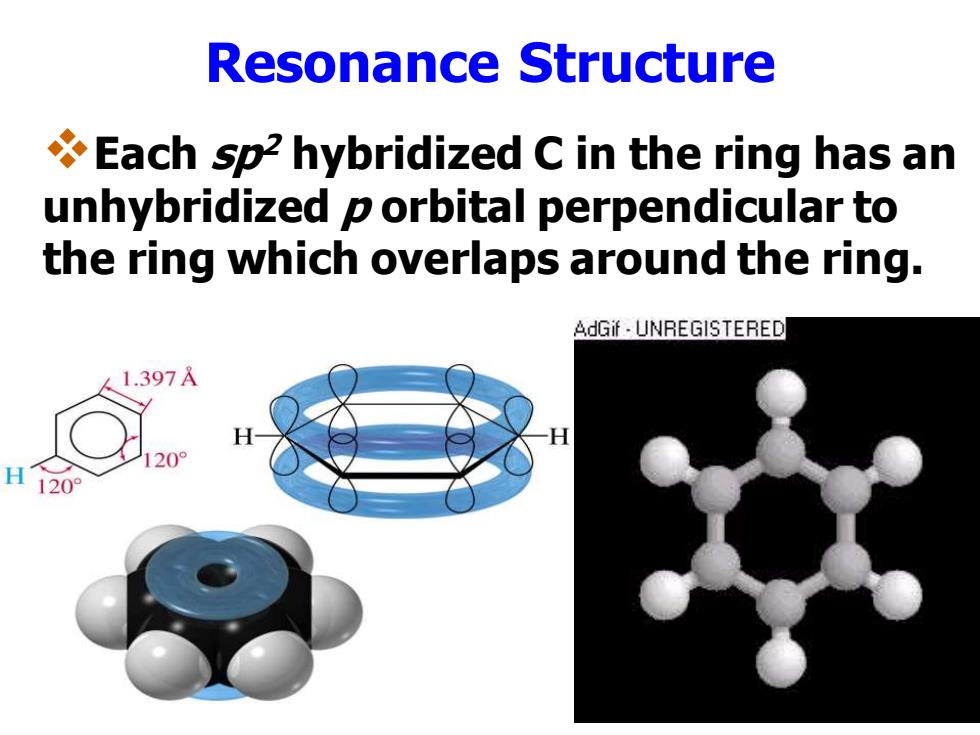
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. AdGif UNREGISTERED 1.397 H120°
Resonance Structure ❖Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring