Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 2 Structure and Properties of Organic Molecules Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©20o3,Prentice Hall
Chapter 2 Structure and Properties of Organic Molecules Organic Chemistry, 5th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall
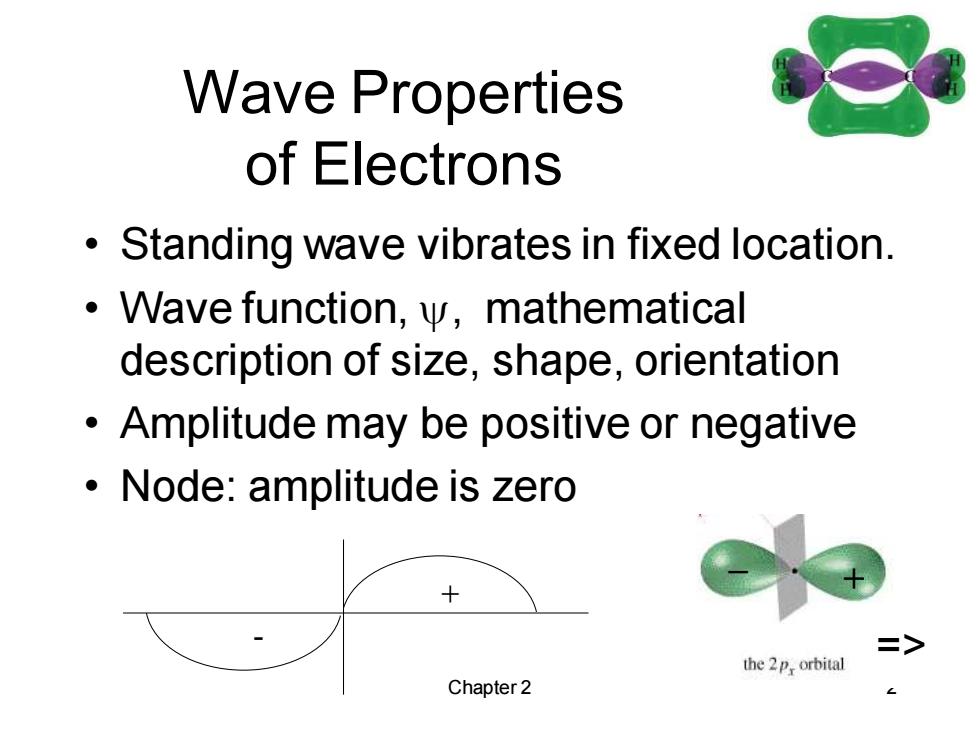
Wave Properties of Electrons Standing wave vibrates in fixed location. Wave function,y,mathematical description of size,shape,orientation Amplitude may be positive or negative Node:amplitude is zero > the 2p,orbital Chapter 2
Chapter 2 2 Wave Properties of Electrons • Standing wave vibrates in fixed location. • Wave function, , mathematical description of size, shape, orientation • Amplitude may be positive or negative • Node: amplitude is zero + _ + - =>
Wave Interactions Linear combination of atomic orbitals >between different atoms is bond formation >on the same atom is hybridization. Conservation of orbitals Waves that are in phase add together. Amplitude increases. Waves that are out of phase cancel out. > Chapter 2 3
Chapter 2 3 Wave Interactions • Linear combination of atomic orbitals ➢between different atoms is bond formation ➢on the same atom is hybridization. • Conservation of orbitals • Waves that are in phase add together. Amplitude increases. • Waves that are out of phase cancel out. =>
Sigma Bonding Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p, or hybridized orbital overlaps. The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals. => Chapter 2 4
Chapter 2 4 Sigma Bonding • Electron density lies between the nuclei. • A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. • The bonding MO is lower in energy than the original atomic orbitals. • The antibonding MO is higher in energy than the atomic orbitals. =>
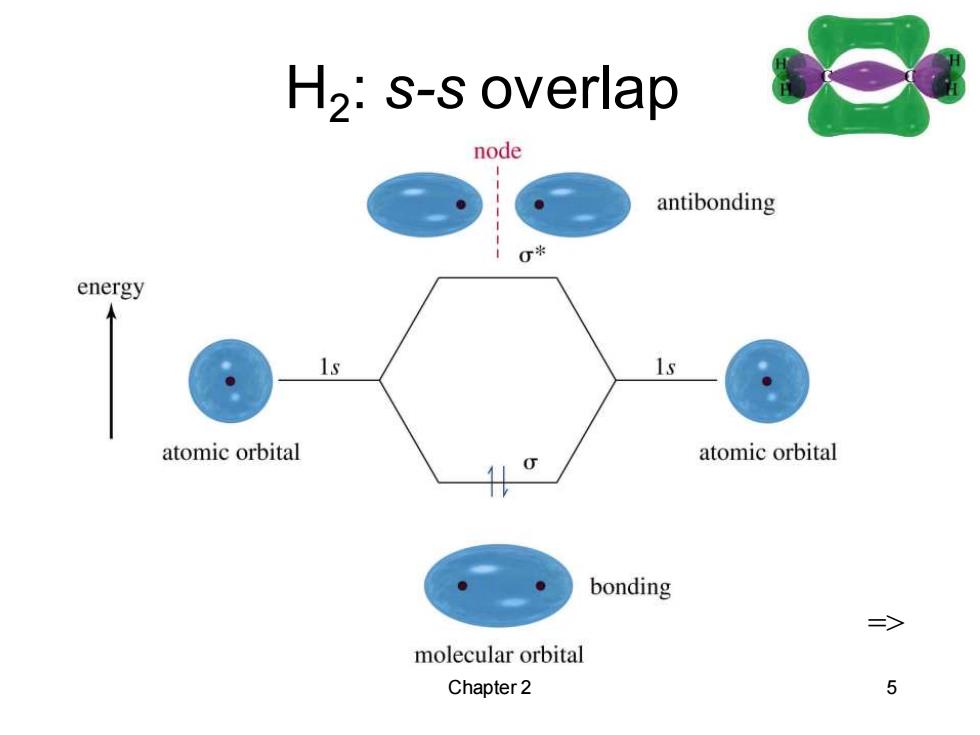
H2:s-s overlap node antibonding 0米 energy atomic orbital atomic orbital bonding => molecular orbital Chapter 2 5
Chapter 2 5 H2 : s-s overlap =>