
Alkenes:Structure and Reactivity Based on McMurry's Organic Chemistry,6th edition
Alkenes: Structure and Reactivity Based on McMurry’s Organic Chemistry, 6th edition
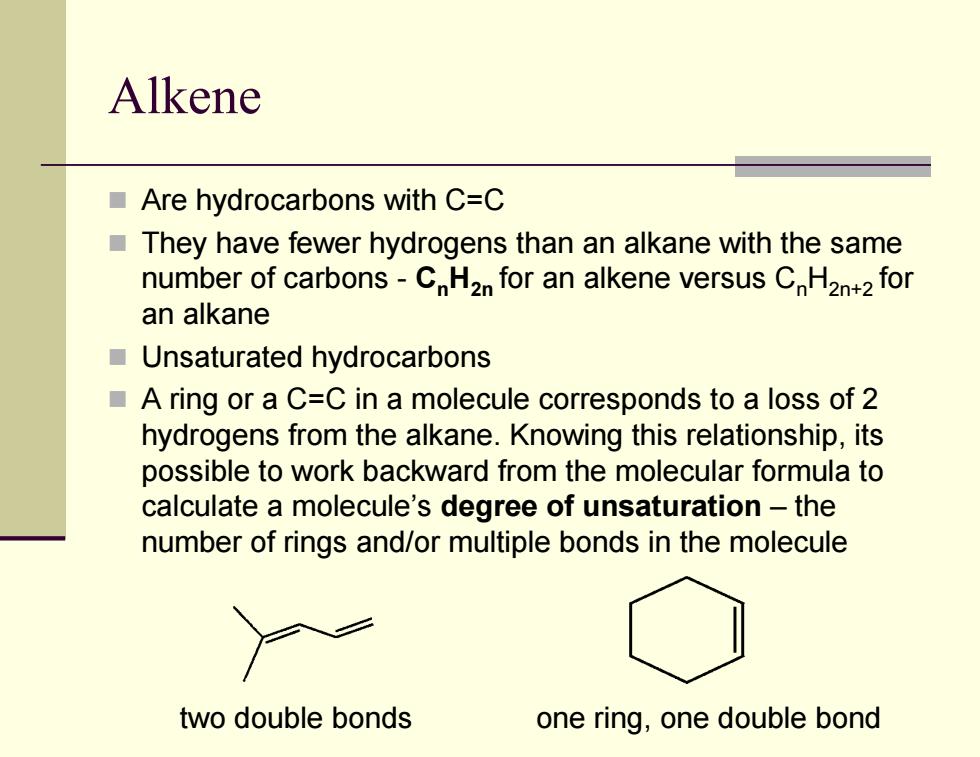
Alkene Are hydrocarbons with C=C They have fewer hydrogens than an alkane with the same number of carbons-CH2n for an alkene versus CH2n+2 for an alkane Unsaturated hydrocarbons A ring or a C=C in a molecule corresponds to a loss of 2 hydrogens from the alkane.Knowing this relationship,its possible to work backward from the molecular formula to calculate a molecule's degree of unsaturation-the number of rings and/or multiple bonds in the molecule two double bonds one ring,one double bond
Alkene Are hydrocarbons with C=C They have fewer hydrogens than an alkane with the same number of carbons - CnH2n for an alkene versus CnH2n+2 for an alkane Unsaturated hydrocarbons A ring or a C=C in a molecule corresponds to a loss of 2 hydrogens from the alkane. Knowing this relationship, its possible to work backward from the molecular formula to calculate a molecule’s degree of unsaturation – the number of rings and/or multiple bonds in the molecule two double bonds one ring, one double bond

Degree of Unsaturation With Other Elements ■ Organohalogens (X:F,CI,Br,I) Halogen replaces hydrogen Replace 2 Br by 2 H BrCH2CH-CHCH2Br = HCH2CH-CHCH2H C.H6Br2 =“C4Hg” One unsaturation: one double bond Add e2004 Thomson-Brooks/Cole Oxygen atoms-if connected by single bonds These don't affect the total count of H's O removed from here H2C=CHCH-CHCH2OH H2C=CHCH=CHCH2-H CsHg0=“CsH3”Two unsaturations: two double bonds 2004 Thomson-Brooks/Cole
Degree of Unsaturation With Other Elements Organohalogens (X: F, Cl, Br, I) Halogen replaces hydrogen Oxygen atoms - if connected by single bonds These don't affect the total count of H's
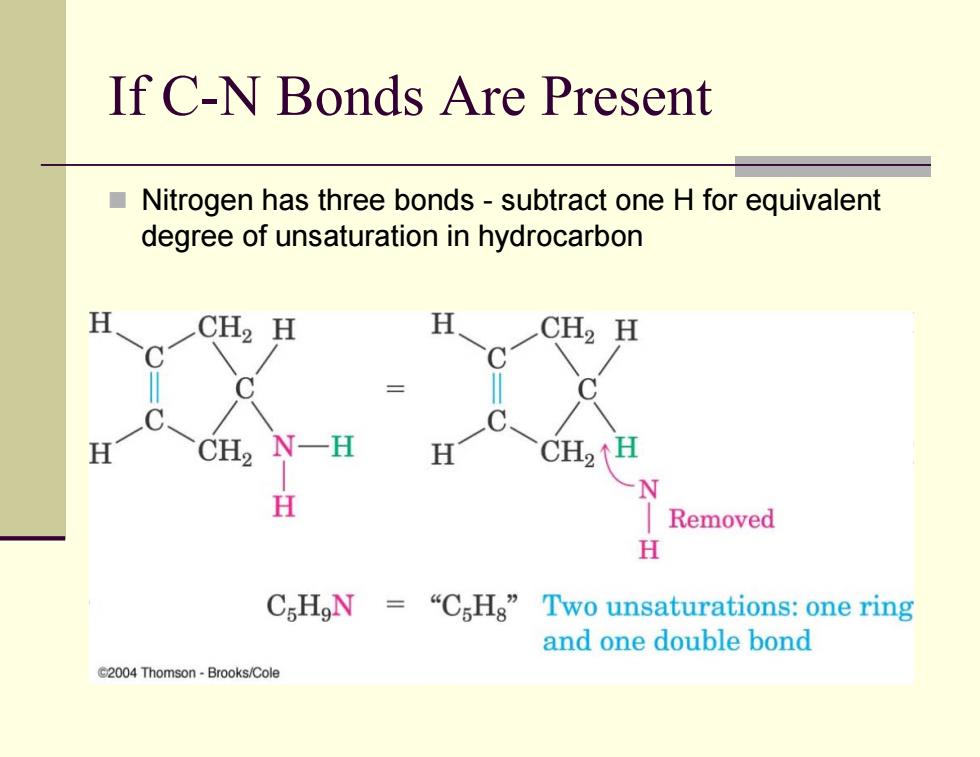
If C-N Bonds Are Present Nitrogen has three bonds-subtract one H for equivalent degree of unsaturation in hydrocarbon H CH2 H H CH2 H H CH2 N一H H CH2 H Removed H CsHgN=“C5Hg”Two unsaturations:one ring and one double bond C2004 Thomson-Brooks/Cole
If C-N Bonds Are Present Nitrogen has three bonds - subtract one H for equivalent degree of unsaturation in hydrocarbon
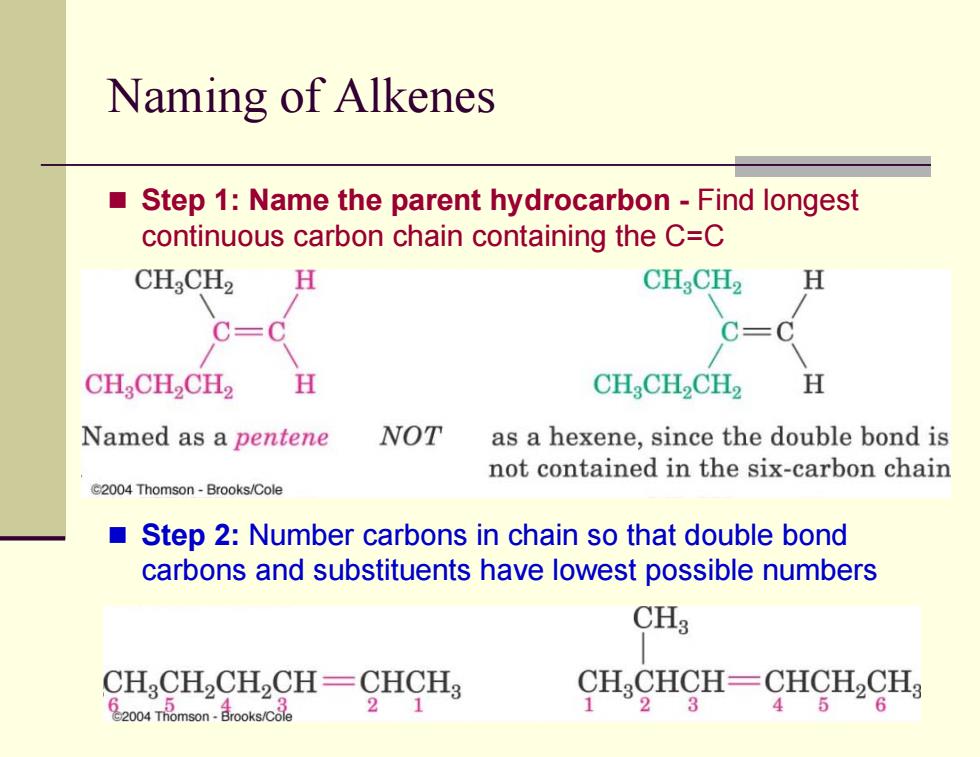
Naming of Alkenes Step 1:Name the parent hydrocarbon-Find longest continuous carbon chain containing the C=C CHCH2 H CH:CH2 H C=C C=C CH2CH2CH2 H CHCH2CH2 H Named as a pentene NOT as a hexene,since the double bond is not contained in the six-carbon chain C2004 Thomson-Brooks/Cole Step 2:Number carbons in chain so that double bond carbons and substituents have lowest possible numbers CH3 CHCH,CH2CH-CHCH3 CH CHCH-CHCH2CH 20o4Tmson- 21 1 23 45 6
Naming of Alkenes Step 1: Name the parent hydrocarbon - Find longest continuous carbon chain containing the C=C Step 2: Number carbons in chain so that double bond carbons and substituents have lowest possible numbers