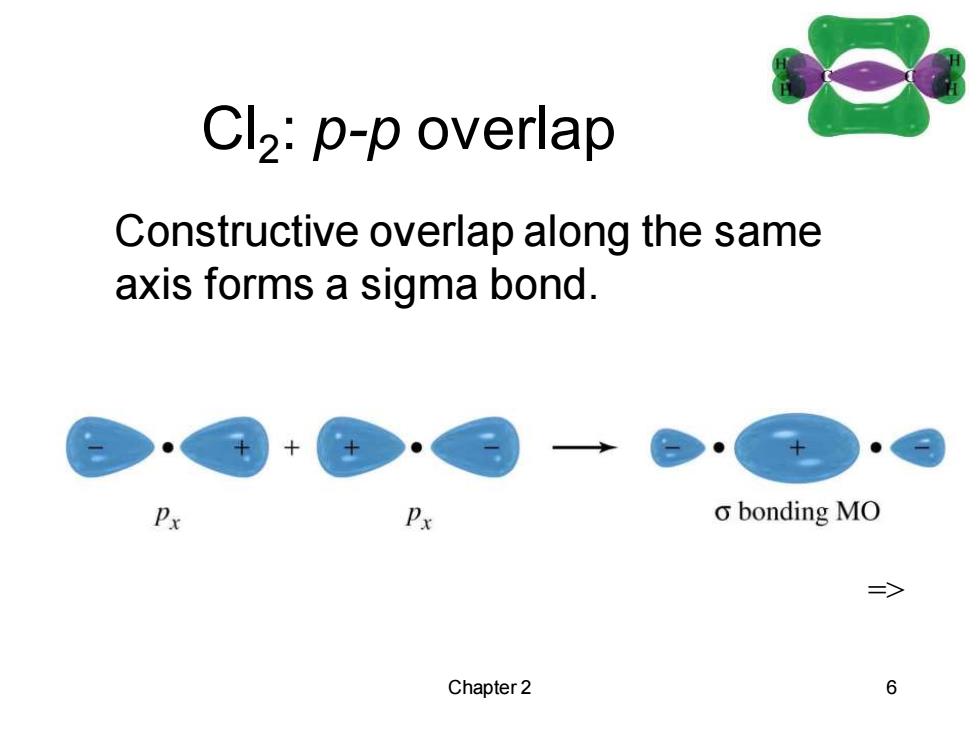
Cl2:p-p overlap Constructive overlap along the same axis forms a sigma bond. Px o bonding MO Chapter 2 6
Chapter 2 6 Cl2 : p-p overlap => Constructive overlap along the same axis forms a sigma bond
HCI:s-p overlap Question: Draw the predicted shape for the bonding molecular orbital and the antibonding molecular orbital of the HCI molecule. Answer:See bottom of page 42 in your text. => Chapter 2 7
Chapter 2 7 HCl: s-p overlap Question: Draw the predicted shape for the bonding molecular orbital and the antibonding molecular orbital of the HCl molecule. Answer: See bottom of page 42 in your text. =>
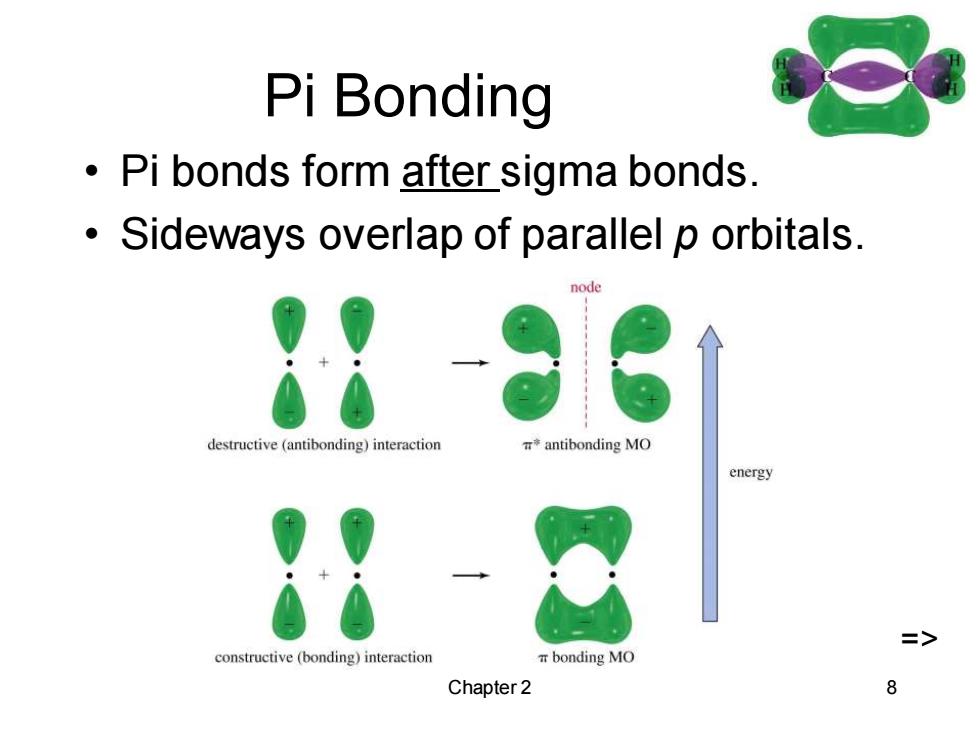
Pi Bonding Pi bonds form after sigma bonds. Sideways overlap of parallel p orbitals. node destructive (antibonding)interaction *antibonding MO energy constructive (bonding)interaction a bonding MO Chapter 2
Chapter 2 8 Pi Bonding • Pi bonds form after sigma bonds. • Sideways overlap of parallel p orbitals. =>
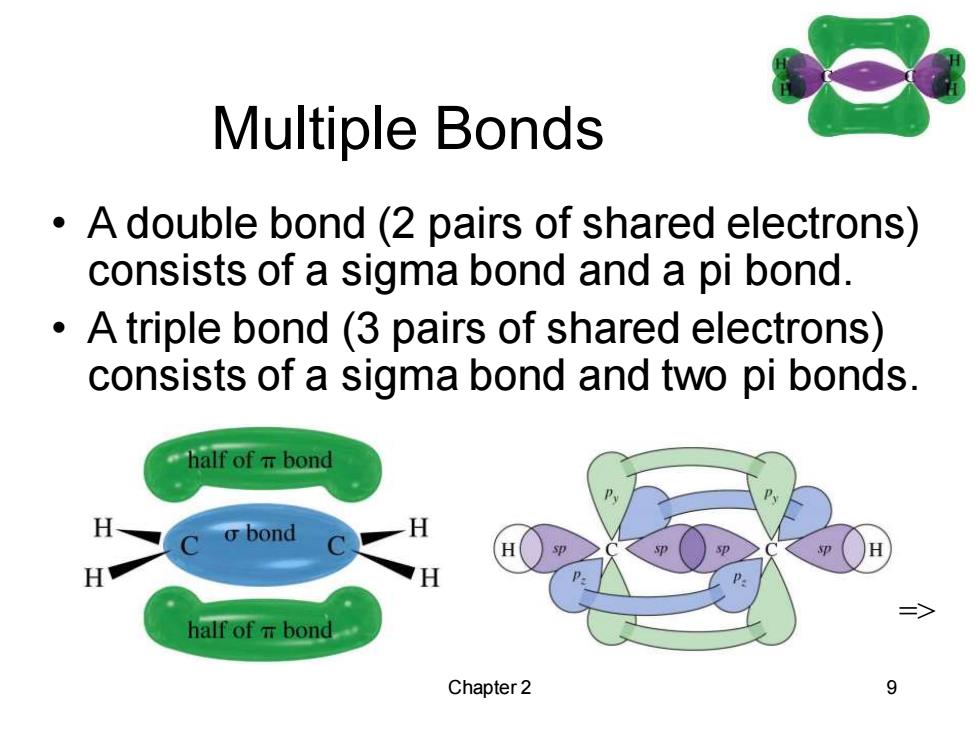
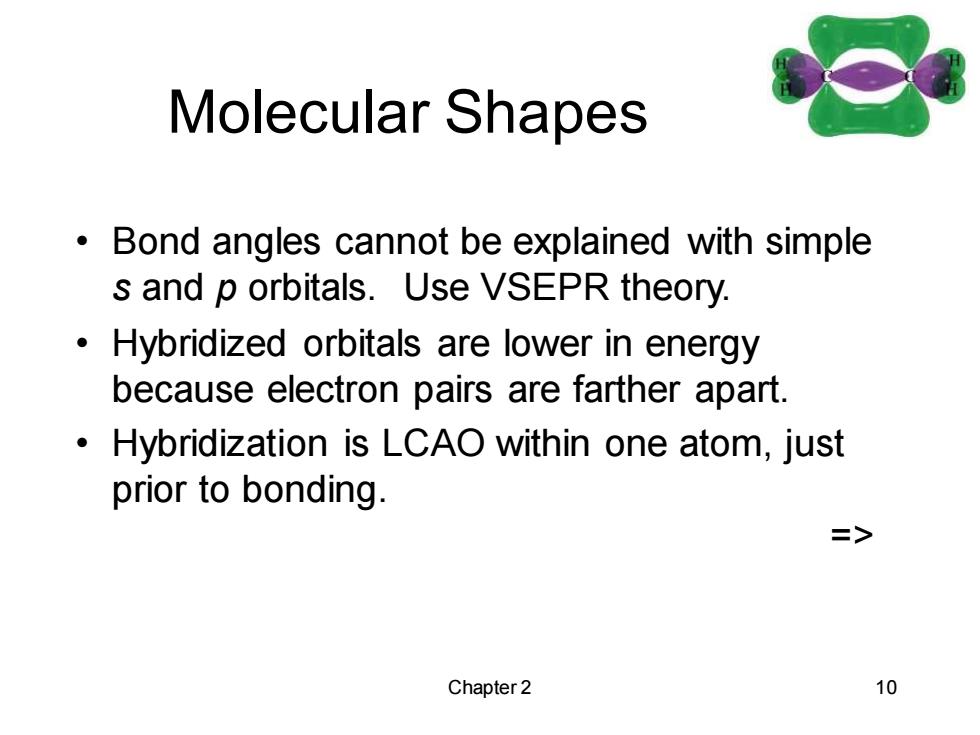
Multiple Bonds A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds. half of T bond C o bond H H H half of T bond Chapter 2 9
Chapter 2 9 Multiple Bonds • A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. • A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds. =>
Molecular Shapes Bond angles cannot be explained with simple s and p orbitals.Use VSEPR theory. Hybridized orbitals are lower in energy because electron pairs are farther apart. Hybridization is LCAO within one atom,just prior to bonding. => Chapter 2 10
Chapter 2 10 Molecular Shapes • Bond angles cannot be explained with simple s and p orbitals. Use VSEPR theory. • Hybridized orbitals are lower in energy because electron pairs are farther apart. • Hybridization is LCAO within one atom, just prior to bonding. =>