
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 3 Structure and Stereochemistry of Alkanes Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 3 Structure and Stereochemistry of Alkanes Organic Chemistry, 6th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall
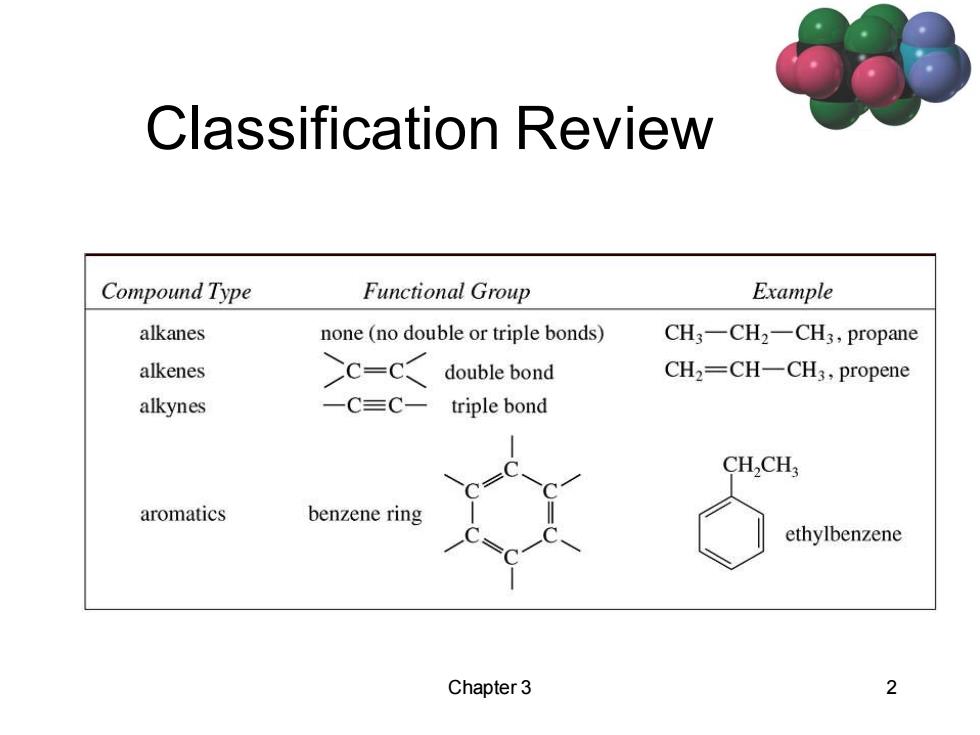
Classification Review Compound Type Functional Group Example alkanes none (no double or triple bonds) CH3一CH2-CH3,propane alkenes >c-c< double bond CH2=CH-CH3,propene alkynes 一C三C一 triple bond CHCH aromatics benzene ring ethylbenzene Chapter 3 2
Chapter 3 2 Classification Review
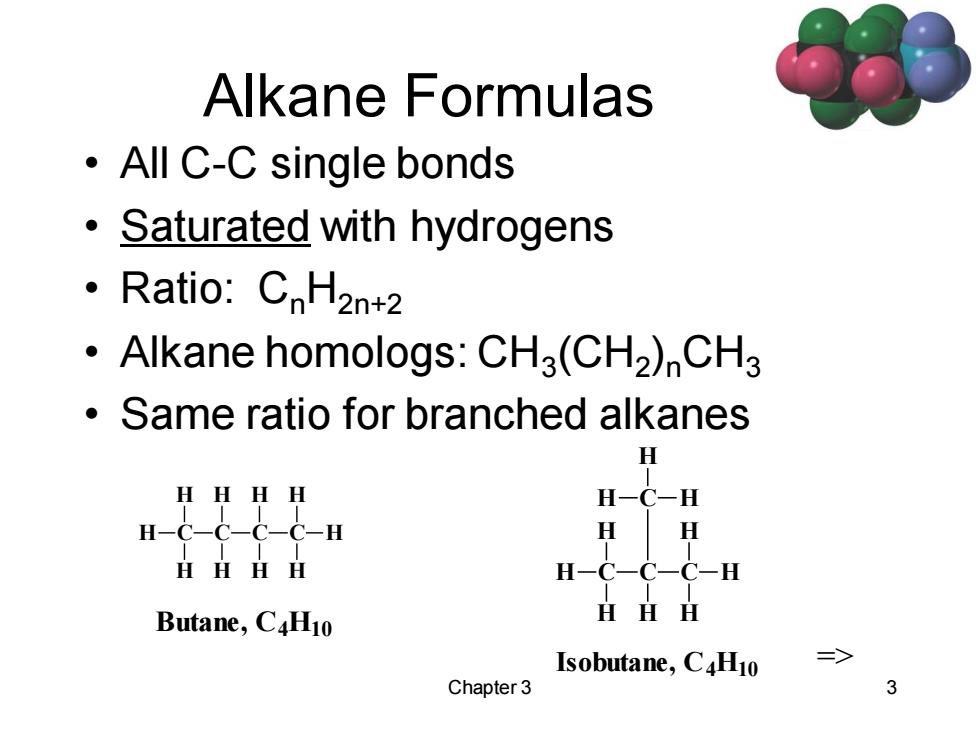
Alkane Formulas ·AllC-C single bonds Saturated with hydrogens 。Ratio::CnH2nt2 Alkane homologs:CH3(CH2)CH3 Same ratio for branched alkanes H HHHH H-C-H H-c-c-c-C-H H H HHHH H-C-C-C-H Butane,C4H10 HHH Isobutane,C4H10 => Chapter 3 3
Chapter 3 3 Alkane Formulas • All C-C single bonds • Saturated with hydrogens • Ratio: CnH2n+2 • Alkane homologs: CH3 (CH2 )nCH3 • Same ratio for branched alkanes => C H C H H H C H H H C H H H Isobutane, C4H10 C H C H H H C C H H H H H H Butane, C4H10

Common Names 。Isobutane,“isomer of butane” Isopentane,isohexane,etc.,methyl branch on next-to-last carbon in chain. Neopentane,most highly branched Five possible isomers of hexane, 18 isomers of octane and 75 for decane! Chapter 3 4
Chapter 3 4 Common Names • Isobutane, “isomer of butane” • Isopentane, isohexane, etc., methyl branch on next-to-last carbon in chain. • Neopentane, most highly branched • Five possible isomers of hexane, 18 isomers of octane and 75 for decane! =>
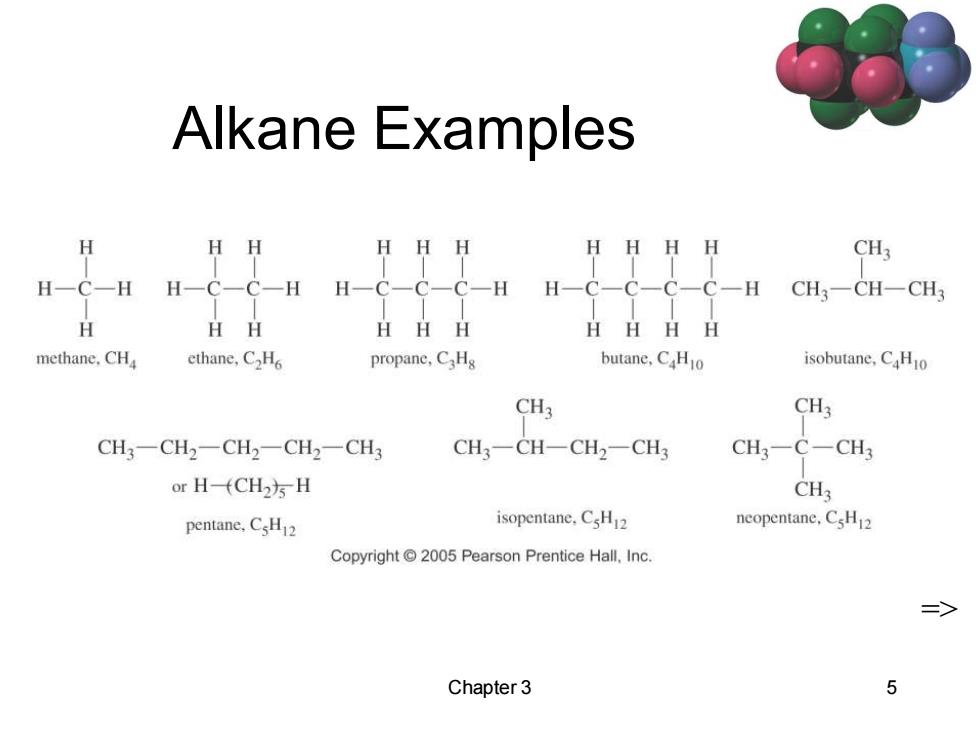
Alkane Examples HH HHHH CH3 H一C-H H-C-C-H H-C-C-C-H H-C- -C- CH3一CH-CH H HH HHH HHHH methane,CHa ethane,C2H6 propane,CHg butane,CHo isobutane,CHo CH3 CH3 CH3-CH2-CH2一CH2一CH3 CH3一CH-CH2-CH3 CH3-C-CH3 or H-fCH2)s H CH3 pentane.CsH2 isopentane.CsH2 neopentane,CsH2 Copyright 2005 Pearson Prentice Hall,Inc. 二> Chapter 3
Chapter 3 5 Alkane Examples =>